Wisconsin - PowerPoint PPT Presentation
Title:
Wisconsin
Description:
Wisconsin Oncology Network (WON) Anne Traynor, MD UWCCC Heme Onc amt_at_medicine.wisc.edu Mary Beth Wims Lynn Volk UWCCC Central Research Office mbwims_at_uwcarbone.wisc.edu – PowerPoint PPT presentation
Number of Views:61
Avg rating:3.0/5.0
Title: Wisconsin
1
Wisconsin Oncology Network (WON)
Anne Traynor, MD UWCCC Heme Onc amt_at_medicine.wisc.
edu Mary Beth Wims Lynn Volk UWCCC Central
Research Office mbwims_at_uwcarbone.wisc.edu lmvolk_at_u
wcarbone.wisc.edu
2
Disclosures
- I, on behalf of the UW Board of Regents, receive
research funding from the pharmaceutical
companies listed. This support pays the salaries
of the UWCCC lung cancer research staff. - Eli Lilly and Company
- Novartis
- Bayer Pharmaceuticals
- I will discuss unapproved use of investigational
agents or combinations.
3
Wisconsin Oncology Network
- Collegial research network developed by the UWCCC
in 1999 - Extend the conduct of investigator-initiated
phase II heme/onc trials through Wisconsin - Offer innovative protocols locally
- Local sites accrue roughly 1/3 to 1/2 of patients
- Phase II trials estimate efficacy of a novel
agent/combination in a specific disease site - Safety, tolerability, and dose previously
determined in a Phase I trial - Phase II Prostate cancer, non-Hodgkins
lymphoma, colon cancer, etc. - Single arm phase II usually enrolls 30 50 pts
and compares to historical controls - Newer 2 arm phase II trials randomize to
investigational and control arms (about 40 - 50
per arm) in order to obtain estimates of efficacy
compared to the concurrent control arm - Phase III trials compare novel agent to standard
of care - Outreach of novel anti-cancer treatments and
education is an important mission of the UW
Carbone Cancer Center
4
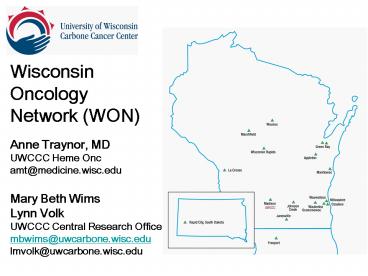
WON Members
- Aurora Health Care System
- Bellin Cancer Team
- Green Bay
- Cancer Center at Aspirus Hosp
- Wausau
- Columbia-St. Marys
- Milwaukee
- Dean Clinic
- South Central Wisconsin
- Ferguson Cancer Center
- Freeport, IL
- Fox Valley Hematology/Onc
- Green Bay Oncology
- Gundersen Lutheran
- LaCrosse
- Holy Family Cancer Center
- Manitowoc
- Marshfield Clinic
- Medical College of Wisconsin
- Mercy Health
- Janesville
- Regional CC of Waukesha and
- Oconomowoc
- UWCCC UWHC and 1 South Park in Madison
- UWCCC Partners
- Johnson Creek
- Wisconsin Rapids
- Vucurevich Cancer Center
- Rapid City, SD
5
WON Members
- Experienced in clinical oncology research
- All conduct NCI Cooperative Group studies
- NCI R01 grant-funded research site
- Vucurevich CC in Rapid City, SD
- Access to cancer care for Native American
populations - NCI Community Clinical Oncology Program (CCOP)
- Marshfield Clinic, Green Bay Oncology, Gundersen
Lutheran - NCI Community Cancer Center Program
- Gundersen Lutheran, Columbia/St. Marys, Regional
CC of Waukesha/Oconomowoc - Multiple sites have received ASCO awards
6
WON April 2006 8 members
WON Sept 2011 17 members
7
Open WON Studies
- Bortezomib temsirolimus in non-Hodgkins
lymphoma - Tim Fenske, MD, PI from Medical College of WI
- Intra-pt dose escalation sorafenib in Kras
positive non-small cell lung cancer - Bendamustine rituximab in chronic lymphocytic
leukemia - High dose estradiol in metastatic breast cancer
- Dose dense docetaxel cyclophosphamide in
adjuvant treatment of resected breast cancer - Panobinostat in I131-refractory thyroid cancer
8
Up-coming WON Studies
- Docetaxel /- suramin in non-small cell lung
cancer - Rafael Santana-Davila, MD, PI from MCW
- Thoracic radiation with adaptive image guidance
in limited stage small cell lung cancer - Bendamustine/rituximab/lenalinomide in chronic
lymphocytic leukemia - Afatinib and paclitaxel in HER2 metastatic
breast cancer
9
Recently Completed WON Studies
- Bevacizumab, lenalidomide, dex in multiple
myeloma - NCI study (UWCCC funded)
- Bortezomib/doxorubicin/ALCAR in multiple myeloma
- Vincristine-CVAD rituximab in mantle cell
lymphoma - Bortezomib doxorubicin/ALCAR in breast cancer
- Funded by an NIH R21
- Vorinostat in non-small cell lung cancer
- NCI study (UWCCC funded)
- Vorinostat bortezomib in non-small cell lung
cancer - Oxali, capecitabine, and sorafenib in pancreas
cancer - Lapatinib and capecitabine for colorectal cancer
- Oxaliplatin, 5FU, and capecitabine in colorectal
cancer - Docetaxel /- doxercalciferol in prostate cancer
10
Correlative Assays in WON Trials
- Bevacizumab, lenalidomide, dex in multiple
myeloma - Specimens collected from WON sites
- Plasma at baseline and through treatment
- Shipped to UWCCC on dry ice
- Bone marrow aspirates
- Shipped to UWCCC in FedEx cold pack
- Findings
- Baseline MIP1a levels predicted response to
therapy
11
WON
- Phase II investigator-initiated heme/onc trials
- Novel agents may be provided by industry or the
NCI - Funding for administration of the study provided
by industry, NCI grant, or UWCCC via
peer-reviewed Pilot Project grant - May be developed from any site
- Sites may (or may not) open any study
- No minimum accrual requirement
- WON is wholly funded by the UWCCC NIH Cancer
Center Support Grant - UWCCC supports all aspects of conducting and
regulating WON trials (1.5 FTEs) - No membership fee for local sites to join WON
- WON does not receive industry support
12
WON as a Network
- All sites sign an Affiliation Agreement for the
Conduct of Clinical Research with UW Board of
Regents when they join WON - This subcontract covers all activities pertaining
to all WON studies, present and future - Wisconsin IRB Consortium
- UW, MCW, Marshfield Clinic, Aurora Healthcare
- Remaining 3 sites defer to first IRB that
approved study - Encouraging other site IRBs to defer to UW IRB
- Drug distribution
- Either from UW Pharmaceutical Research Center or
directly to WON sites from industry sponsor
13
WON as a Network
- All clinical trial data are entered into UW
on-line database (Oncore) and analyzed in final
results - Sites receive Oncore training from UWCCC
- All studies must follow UWCCC Data and Safety
Monitoring Plan - UWCCC DSM Committee monitors all studies
- On-site audit every 3 yrs
- All accruing sites are listed as co-authors in
publications - Communication
- Spring and Fall half-day meetings in Madison
- Educational lunch time talk by UW faculty
- No registration fee to attend
- Monthly conference calls
- Weekly email updates
14
WON Since 1999
- 50 trials
- Vast majority industry-sponsored IITs
- Others R01, R21, NCI agent with UWCCC support
- 2 have originated from MCW, 1 from Gundersen
- 1141 patients enrolled
- About to open our first randomized phase II study
- 400 enrolled at non-UWHC sites (35)
- 16 articles in peer-reviewed journals
- 23 posters or abstracts
15
J Thorac Oncol 20094522-6
Chang JE, Peterson C, Choi S, Eickhoff JC, Kim K,
Yang DT, Gilbert LA, Rogers ES, Werndli JE, Huie
ME, McFarland TA, Volk M, Blank J, Callander NS,
Longo WL, Kahl BS. VcR-CVAD induction
chemotherapy followed by maintenance rituximab in
mantle cell lymphoma A Wisconsin Oncology
Network study. Br J Haematol Aug 16, 2011 Epub
ahead of print.
16
WON and the Mayo Clinic Phase II
Consortium (MCP2C)
- WON sites may open any MCP2C study that is open
at UWCCC - MCP2C is one of 9 NCI-funded consortia that
studies NCI-developed drugs - Highly competitive international solicitation
process - This partnership allows WON sites access to novel
agents that have been evaluated by the NCI - Examples of agents/studies
- Cediranib in acute leukemia and myelodysplasia
- OSI-906 in breast cancer and small cell lung
cancer - Veliparib and carboplatin in metastatic breast
cancer
17
Mayo Clinic Phase 2 Consortium
IA
CO
Hong Kong
Singapore
South Korea
18
WON Challenges
- Since the economic downturn, the pharmaceutical
industry has markedly reduced financial support
for investigator-initiated trials, including
limiting the availability of drugs for clinical
trials. - Reduced NIH/NCI funding for the CCSG
- Many academic networks charge membership fees
- Auditing multicenter trials
- Annual virtual audit
- Network-wide IRB deferral system
19
WON Summary
- Highly collegial, efficient, motivated,
experienced clinical trial network that extends
state-of-the-art oncology research throughout the
state - Growing membership, increasing rate of accrual
- Randomized phase II trials possible
- Four largest members defer to single IRB (WIC)
- Drug distribution by UW Pharmacy
- On-line clinical data entry and analyses
- Collection of specimens for correlatives possible
- Data and safety monitoring plan in place
- We need more novel drugs to study





























![❤️[READ]✔️ Wisconsin Supper Clubs: An Old-Fashioned Experience PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10078052.th0.jpg?_=20240713063)
