SFC and SF Extraction - PowerPoint PPT Presentation
Title:
SFC and SF Extraction
Description:
Gel Electrophoresis Slab for proteins and DNA Cooling/sieving mechanism polyacrylamide Capillary isotachophoresis Capillary electrochromatography Micellar ... – PowerPoint PPT presentation
Number of Views:123
Avg rating:3.0/5.0
Title: SFC and SF Extraction
1
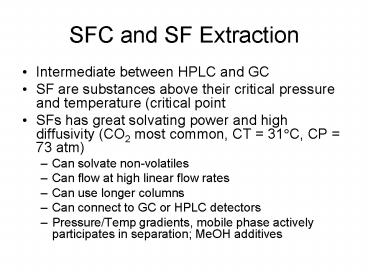
SFC and SF Extraction
- Intermediate between HPLC and GC
- SF are substances above their critical pressure
and temperature (critical point - SFs has great solvating power and high
diffusivity (CO2 most common, CT 31?C, CP 73
atm) - Can solvate non-volatiles
- Can flow at high linear flow rates
- Can use longer columns
- Can connect to GC or HPLC detectors
- Pressure/Temp gradients, mobile phase actively
participates in separation MeOH additives
2
Capillary Electrophoresis
- Small open tubular capillary
- High voltage
- Electrolyte
- Small sample plug
- Electrophoretic mobility
- m (q/f)(E)
- detector
3
Why cap electrophoresis?
- Separation of ions
- High separation efficiency
- No stationary phase
- Plug profile
- Only longitudinal diffusion term
- Very high plate numbers, 106
4
Experimental set-up
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
EO
ions
- ions
Small sample plug
Electrolyte buffer
5
Mobility
- Combination of electrophoritic flow and
electrosmotic flow - v vep veo
- vep mE
- Veo is governed by the pH and ionic strength of
buffer
6
v vep veo
5 2 3
3 0 3
N
1 -2 3
-
N
-
7
Challenges
- Need a small sample size (concentrated sample)
- Pre-concentrate large sample
- stacking
- Can not separate neutrals
- Add micelles
- Pre-concentrate large sample
- stacking
8
Stacking
- Fill capillary with buffer of weaker ionic
strength, 0.10 NaCl - Add a large plug of sample with higher ionic
strength - Create a sandwich by adding weaker buffer
- Apply voltage for a brief while
- Change leads and apply voltage for a while
- Change back and start analysis
9
Fill with 0.1 M NaCl
-
10
Fill with sample
0.01 M NaCl
0.1 M NaCl
-
11
Apply voltage
0.01 M NaCl
0.1 M NaCl
-
12
Switch Electrodes
0.01 M NaCl
0.1 M NaCl
-
13
Switch Back and begin separation
0.01 M NaCl
0.1 M NaCl
-
14
Different Types of CE
- Capillary Zone Electrophoresis
- Small ions
- Capillary isoelectric focusing
- Amphoteric compounds
- Cap. Gel Electrophoresis
- Slab for proteins and DNA
- Cooling/sieving mechanism
- polyacrylamide
- Capillary isotachophoresis
- Capillary electrochromatography
- Micellar Electrokinetic chromatography
15
CZE
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
EO
ions
- ions
Small sample plug
Electrolyte buffer
16
Capallary Gel Electrophoresis
- Slab Gel Electrophoresis for proteins and DNA
- Cooling/sieving mechanism
- Polyacrylamide
- Some capillary applications, as well
- 2 D Gel Electrophoresis
- Separates by size and pI
17
(No Transcript)
18
Capillary isoelectric focusing-CIEF
- Separation of amphoteric species such as a
protein - pH gradient established
- A protein will move along the gradient until they
reach a pH that correspond to its pI, the pH
where the average charge is zero - Resolution, ?0.2 pI units
- Mobilization of the bands
19
CIEF
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
H ions
OH- ions
Sample and ampholytes
pH 2
pH 12
20
Forming the bands
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
H ions
pI 4.1
pI 8.3
OH- ions
Sample and ampholytes
pH 2
pH 12
21
Mobilizing the bands
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
H ions
pI 4.1
pI 8.3
OH- ions
Add NaCl
Cl- ions
Sample and ampholytes
pH 2
pH 12
22
Capillary Isotachophoresis
- Sandwich sample between a leading and a lagging
buffer - Leading buffer is faster than each of the
analytes - Lagging buffer is slower than each of the
analytes - Analytes form bands between buffers
- Once band form they whole solution in the
capillary moves at a constant velocity
23
Mobilizing the bands
20 kV Power Supply
-
Fused silica Capillary 50 mm ID
UV detector
flow
Leading buffer
Lagging buffur
pH 12