Acid Rain Clicker Quiz - PowerPoint PPT Presentation
1 / 20
Title:
Acid Rain Clicker Quiz
Description:
Acid leaches nutrients from the soil, ... Sulfur dioxide only Nitrogen oxides only Carbon dioxide Sulfur and nitrogen oxides Why is rain naturally slightly acidic? – PowerPoint PPT presentation
Number of Views:250
Avg rating:3.0/5.0
Title: Acid Rain Clicker Quiz
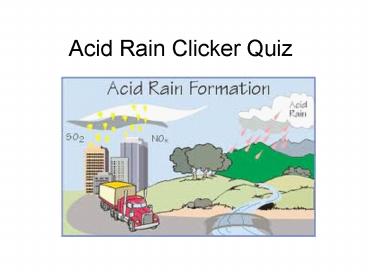
1
Acid Rain Clicker Quiz
2
What is acid precipitation?
- Any precipitation with a pH of 5.6
- Precipitation with a pH of less that 5.6
- Precipitation that is not neutral
- Precipitation with a high pH
3
Which is a direct effect of acid rain on
vegetation?
- Acid leaches nutrients from the soil, making
plants grow poorly - Acid damages the foliage of plants
- Acid makes the plants grow faster
4
What is an indirect affect of acid rain on
Aquatic Life?
- Acid rain burns the fish
- Acid rain leaches Aluminum from soil which
affects fish reproduction. - Acid rain makes the pH of the lake lower.
- Acid rain makes fields acidic
5
(No Transcript)
6
Which animal is the most sensitive to acid rain?
- Trout
- Bass
- Perch
- Frogs
- Clams
7
How does Acid rain affect buildings, structures
and monuments?
- Acid rain does not affect buildings.
- Acid rain dissolves marble and limestone building
materials and causes corrosion of metals. - Acid rain leaches nutrients from the soil.
- Acid rain affects peoples lungs.
8
- How does acid rain indirectly affect plants?
- The acids burn the roots of plants
- The acids lower the rate of germination of seeds
- The acids leach nutrients from the soil
- The acids make the soil acidic.
9
- What are the major sources of air pollutants
that cause acid rain? - Burning coal in power plants and industry and
transportation - Burning coal only
- Driving cars and transportation only
- Burning wood
10
- What are the primary pollutants that cause acid
rain? - Sulfur dioxide only
- Nitrogen oxides only
- Carbon dioxide
- Sulfur and nitrogen oxides
11
Why is rain naturally slightly acidic?
- Rain water is slightly acidic because sulfur
dioxide in the air dissolves in rain water to
form a weak acid. - Rain water is slightly acidic because carbon
dioxide in the air dissolves in rain water to
form a weak acid. - Rain water is slightly acidic because nitrogen
dioxide in the air dissolves in rain water to
form a weak acid.
12
Which process produces the most sulfur dioxide in
the USA?
- Coal power plants
- Industry
- Transport
13
Where is the acid deposition the most severe?
- North Eastern USA
- North Western USA
- Southern USA
- Northern USA
14
What are the secondary pollutants in acid rain?
- Nitrogen oxides react to form nitric and sulfuric
acids. - Nitrogen oxides react to form nitric and nitrous
acids. Sulfur oxides react to from sulfuric and
sulfurous acids. - Sulfur oxides react to form nitric, nitrous,
sulfuric and sulfurous acids.
15
Evaluate the catalytic converter as a way to
lower acid rain.
- The catalytic converter reduces sulfur dioxide
emissions - Nitrogen oxide emissions are lowered, but not
100 - Carbon dioxide emissions are lowered.
- It is an inexpensive solution
16
Which are methods to reduce acid rain?
- Catalytic converters, liming lakes, and alkaline
scrubbers - Alkaline scrubbers, catalytic converters,
removing sulfur from coal. - Liming lakes, catalytic converters, and removing
sulfur from coal
17
Evaluate liming lakes as a way to mitigate the
effects of acid rain.
- The pH is restored, but some species do not
return to the lake. - The pH can be raised, but not high enough to make
a difference - The pH can be raised, but the process is
expensive, and some species do not recover.
18
What substances are converted by the catalytic
converter?
- Carbon dioxide, oxygen, and unburned hydrocarbons
are converted into carbon monoxide and water. - Carbon, unburned hydrocarbons, nitrogen oxides
are converted into carbon dioxide, nitrogen and
water. - Carbon monoxide, unburned hydrocarbons, nitrogen
oxides are converted into carbon dioxide,
nitrogen and water.
19
Which catalytic converter is most effective?
- A B C
20
Which power source produces the most air
pollution?
- Nuclear
- Hydroelectric
- Wind
- Solar
- Coal
- Natural gas