Titrations - PowerPoint PPT Presentation
1 / 11
Title:
Titrations
Description:
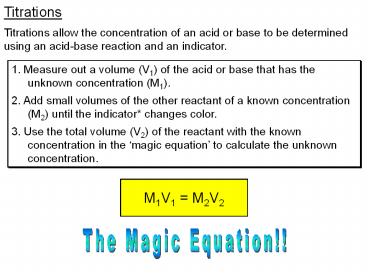
Titrations Titrations allow the concentration of an acid or base to be determined using an acid-base reaction and an indicator. 1. Measure out a volume (V1) of the ... – PowerPoint PPT presentation
Number of Views:199
Avg rating:3.0/5.0
Title: Titrations
1
Titrations
Titrations allow the concentration of an acid or
base to be determined using an acid-base reaction
and an indicator.
1. Measure out a volume (V1) of the acid or base
that has the unknown concentration (M1). 2. Add
small volumes of the other reactant of a known
concentration (M2) until the indicator changes
color. 3. Use the total volume (V2) of the
reactant with the known concentration in the
magic equation to calculate the unknown
concentration.
The Magic Equation!!
2
Indicators
- Indicators are organic dyes that are also weak
acids or weak bases. - The color of the dye depends upon the pH of the
solution. - The indicator will change colors at the pH that
corresponds to its own equivalence point. - Therefore, it is important to pick an indicator
that changes color very close to the pH at the
equivalence point for the titration. - Since the color change does not exactly match the
equivalence point, it is called the ENDPOINT of
the titration.
3
Indicators organic dyes whose color depends upon
the pH of the solution.
4
Examples
1) 25 mL of HCl are titrated with 12.5 mL of 1.0
M NaOH. What is the concentration of the HCl?
M1V1 M2V2
M1(25 mL) (1.0 M)(12.5 mL)
2) 10 mL of NaOH are titrated with 10 mL of 1.0 M
H2SO4. What is the concentration of the NaOH?
H2
n1M1V1 n2M2V2
H1 2 H2SO4
(1)M1(10 mL) 2(1.0 M)(10 mL)
5
Why does the Magic Equation work?
Recall that Molarity moles/volume of soln.
MAVA MBVB
- The Magic Equation gives the volume at which
the number of moles of H exactly equal the
number of moles of OH-.
6
1. What is the pH of 0.100 M HCl?
pH 1
2. What is the pH of 0.100 M NaOH?
pH 13
3. What is Veq?
Veq 50.0 mL
Note The ONLY time the pH 7 at the equivalence
point is during strong acid strong base
titrations.
7
pKa 4.9
Veq
Ka 1.3 x 10-5
The pH at Veq for a weak acid is greater than
7. pKa pH at ½ Veq
8
pKa 9.0
pKb 5.0
Kb 1.0 x 10-5
The pH at Veq for a weak base is less than 7. pKa
pH at ½ Veq
9
Buffers solutions that resist changes in pH
Buffers are mixtures of either
- A weak acid and the salt of its conjugate base
OR
- A weak base and the salt of its conjugate acid
Buffers will maintain a pH that is 1 pH unit of
their pKa
Example Acetic acid has a Ka 1.8 x 10-5
pKa -log(Ka) -log(1.8 x 10-5) 4.74
An acetic acid/sodium acetate mixture will buffer
a solution at a pH of 4.74.
10
Titration of Acetic Acid with NaOH
14
13
12
11
10
9
8
pH
7
6
pH 4.74
5
4
3
2
1
0
0
10
20
30
40
50
Volume NaOH added (mL)
11
An important buffer example The pH of blood must
be maintained at 7.4 0.2 or death may occur.
There are two main buffering equilibria
H2CO3 H2O ? HCO3-1 H3O
Ka 4.3 x 10-7 pKa 6.4
H2PO4-1 H2O ? HPO3-2 H3O
Ka 6.2 x 10-7 pKa 7.2
Example What is the pH of a solution containing
0.2 M H2CO3 and 0.4 M NaHCO3?
H3O 2.15 x 10-7 M
pH 6.67