Carboxylic Acids - PowerPoint PPT Presentation
1 / 43
Title:
Carboxylic Acids
Description:
Carboxylic Acids. nomenclature. Carboxylic Acids. nomenclature. Carboxylic Acid Derivatives ... When naming, the acid section is always named last (it has the ... – PowerPoint PPT presentation
Number of Views:38
Avg rating:3.0/5.0
Title: Carboxylic Acids
1
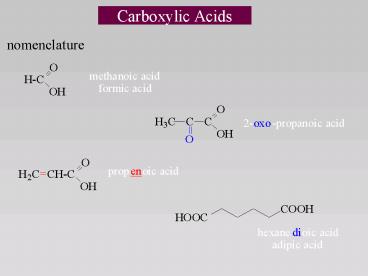
Carboxylic Acids
- nomenclature
2
Carboxylic Acids
- nomenclature
3
Carboxylic Acid Derivatives
- Removal of the OH from the carboxylic acid
- acyl group
4
Carboxylic Acid Derivatives
- further........
5
Carboxylic Acid Derivatives
- therefore....
Acid Chlorides
6
Carboxylic Acid Derivatives
Acid Anhydrides
7
Carboxylic Acid Derivatives Salts Esters
- When naming, the acid section is always named
last (it has the highest priority), and the
suffix is changed - from -ic to ate
8
Carboxylic Acid Derivatives Amides
- Substitute -oic or -ic for amide
9
- For substituted amides....
10
Carboxylic Acid Derivatives Imides
- Imides Amides derived from dicarboxylic acids
11
Carboxylic Acid Derivatives Nitriles
- nitriles are carboxylic acid derivatives as they
can be hydrolysed to amides and subsequently,
acids
12
Carboxylic Acid Derivatives Physical Properties
- Carboxylic acids are generally water soluble
- salts are more soluble (ionic)
- strongly hydrogen bonded, exist as dimers(even
in the gas phase)
13
Carboxylic Acid Derivatives Acidity
- Note Inductive effect electron withdrawing
groups increase acidity. e.g. F-CH2-COOH pka
2.59 - (F withdraws electron density from carboxylic
acid group enables H to be removed more
easily. - Throughbond effect i.e. the further away, the
less influence - electron donating groups decrease acidity.
14
Synthesis of Carboxylic Acids (Review)
- a) Oxidation Reactions
- i) Primary alcohols and aldehydes
- ii) Oxidative Cleavage of Alkenes (see 1st year
notes)
O
eg Jones Reagent Tollens Reagent (AgNO3,
NH4OH)
carboxylic acids
15
Synthesis of Carboxylic Acids
- iii) Synthesis of benzoic acids
- oxidation of any alkyl benzene
16
Synthesis of Carboxylic Acids
- b) Hydrolysis Reactions
- i) hydrolysis of nitriles
- ii) hydrolysis of other acid derivatives (eg
esters, acid chlorides etc etc etc)
17
Synthesis of Carboxylic Acids
- c) Carboxylation of Grignard Reagents
addition of CO2 to Grignard reagents
18
Synthesis of Carboxylic Acids
- carboxylation reactions (continued)
- mechanism...
- can also use organolithium species(replacing
Grignard reagent) - NOTE addition of one extra carbon to original
molecule
19
Reactions of Carboxylic Acids
- Summary
20
Reactions of Carboxylic Acids
- a) ?-substitution
- Bromination The Hell-Volhard-Zelinski
Reaction - via nucleophilic substitution of Br-, forming the
acid bromide
21
Reactions of Carboxylic Acids
- b) Decarboxylation
- heavy metal with a halogen
22
Reactions of Carboxylic Acids
- c) Deprotonation
- carboxylic acids are usually sufficiently acidic
for NaOH to deprotonate - d) Reduction
- use LiAlH4 (i.e NOT NaBH4 sodium borohydride)
23
Reactions of Carboxylic Acids
- reduction (continued)
- borane reacts faster with carboxylic acids
(relative to nitro groups) and therefore
selectivity is achieved - LiAlH4 would also reduce nitro group
24
Reactions of Carboxylic Acids
- e) nucleophilic Acyl Substitution
- these derivatives are usually prepared from the
acid chloride (rather than the acid) as the acid
chlorides are more reactive
25
Reactions of Carboxylic Acids
the chloro is a good leaving group ? molecule
susceptible to nucleophilic attack
- acid chlorides.....
- can use PCl3 or oxalyl chloride (Cl-CO-CO-Cl)
instead of thionyl chloride.
26
Mechanism of Nucleophilic Acyl Subsitution
- 1st step is the rate determining step
27
Mechanism of Nucleophilic Acyl Subsitution
- example
tetrahedral intermediate
28
Nucleophilic Acyl Subsitution Reactivity
- Steric factors the more sterically hindered,
the less reactive - Electronic Factors more strongly favour
polarized derivatives (more
reactive)electronegative halogen polarizes
the carbon more strongly than the alkoxy or amino
group.
29
Preparation of Acid Anhydrides
- 1) Dehydration of the Acid
- water must be removed (to avoid hydrolysis)
- limited use
30
Preparation of Acid Anhydrides
- 2) Nucleophilic Acyl Substitution
- acyl chloride carboxylate anion
- Note suitable for synthesis of unsymmetrical
anhydrides
31
Preparation of Esters
- 1) Direct reaction of carboxylic acids and
alcohols - acid catalysed (increases the acidity of the
carboxylic acid) - all steps are reversible ? need to drive
reaction to the right i.e. remove water or add
excess water - hydrolysis of esters with water (acid
catalysed) is the reverse reaction
32
Preparation of Esters Mechanism
- Same mechanism applies for hydrolysis of esters,
simply in reverse.
33
Preparation of Esters
- 2) Reaction of acid chlorides with
alcohols - Mechanism is nucleophilic acyl substitution
(i.e. the same as previously) - Most general and versatile reaction
- note preparation of alcohol derivatives
practical formation of esters!
34
Preparation of Esters
- 3) Reaction of anhydrides with alcohols
35
Preparation of Esters
- 4) Transesterification
- heat and catalyst (either acid or base)
required - need to push equilibrium towards desired
products
36
Lactones
- Lactones are cyclic esters
- 5- or 6- membered rings are highly favoured
a ?-lactone
37
Amides
38
Peptides and Proteins Amides
- amino acids connected by a seris of amide bonds
- links are called peptides
- macromolecules of this type are proteins
amide or peptide bond
amide bond can be hydrolysed under acidic or
basic conditions digestion
39
Reactions of Amides
- Reduction
- LiAlH4 will reduce the carbonyl bond
95
20 amide
20 amine
80
a lactam (cyclic amide)
cyclic amine
40
Hydrolysis Reactions
- Reaction with water as nucleophile
- Acid chlorides and anhydrides
- react quickly with water producing the
corresponding carboxylic acid (they are often
water soluble)
41
Hydrolysis Reactions
- Esters hydrolysis requires heat and a
catalyst - a) acid catalysed ester hydrolysis
- equilibrium ? use a large excess of water
- mechanism is the reverse of acid catalysed
esterification - b) Saponification base catalysed ester
hydrolysis - hydrolysis of an animal fat, glycerol (3 x acid
esterified with long chain alcoholsfatty acids)
yields soaps
42
Mechanism of Saponification
43
Hydrolysis of Amides and Nitriles
- heat and strong acid or base required
- (similar mechanism to that on previous slide)