The Atmosphere: Oxidizing Medium In Global Biogeochemical Cycles - PowerPoint PPT Presentation
Title:
The Atmosphere: Oxidizing Medium In Global Biogeochemical Cycles
Description:
Only A Narrow UV Window (300-320 nm) for O(1D) Production. 30o equinox. midday. Solar spectrum ... O(1D) production in the troposphere? OH Radical: Main ... – PowerPoint PPT presentation
Number of Views:43
Avg rating:3.0/5.0
Title: The Atmosphere: Oxidizing Medium In Global Biogeochemical Cycles
1
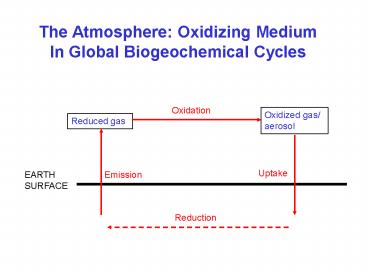
The Atmosphere Oxidizing Medium In Global
Biogeochemical Cycles
Oxidation
Oxidized gas/ aerosol
Reduced gas
Uptake
EARTH SURFACE
Emission
Reduction
2
Only A Narrow UV Window (300-320 nm) for O(1D)
Production
30o equinox midday Solar spectrum
3
O(1D) production in the troposphere?
4
OH Radical Main Tropospheric Oxidant
Primary source
O3 hv ? O2 O(1D) (1) O(1D) M ? O
M (2) O(1D) H2O ? 2OH (3)
Sink oxidation of reduced species
CO OH ? CO2 H CH4 OH ? CH3 H2O HCFC
OH ? H2O
Major OH sinks globally
GLOBAL MEAN OH 1.0x106 molecules cm-3
5
QUESTIONS
1. How would a thinning of the stratospheric
ozone layer affect the source of OH in the
troposphere? 2. How might global warming affect
the source of OH in the troposphere?
6
Global Sources of Methane
Anthropogenic Sources
Natural Sources
7
Global Distribution of MethaneNOAA/CMDL surface
air measurements
8
Historical Trends In Methane
Recent methane trend
Historical methane trend
9
RECENT TREND IN METHANE
10
Global Sources of CO
11
Satellite Measurements of Lower Tropospheric CO
12
Satellite Measurements of Upper Tropospheric CO
13
Global Distribution of CONOAA/CMDL surface air
measurements
14
How are tropospheric OH levels maintained?
Stratosphere
O3
1-2 x1013 moles yr-1
Troposphere
2-4 x1013 moles yr-1
H2O
O3 hv ? OH OH
OH only from strat O3 would be titrated
CO
CH4
6-10 x1013 moles yr-1
3 x1013 moles yr-1
15
GLOBAL DISTRIBUTION OF TROPOSPHERIC OZONE
Climatology of observed ozone at 400 hPa in July
from ozonesondes and MOZAIC aircraft (circles)
and corresponding GEOS-CHEM model results for
1997 (contours).
GEOS-CHEM tropospheric ozone columns for July
1997.
Li et al. 2001
16
NOx EMISSIONS (Tg N yr-1) TO TROPOSPHERE
STRATOSPHERE 0.2
LIGHTNING 5.8
SOILS 5.1
FOSSIL FUEL 23.1
BIOMASS BURNING 5.2
BIOFUEL 2.2
AIRCRAFT 0.5
17
MAPPING OF TROPOSPHERIC NO2FROM THE GOME
SATELLITE INSTRUMENT (July 1996)
Martin et al. 2002
18
LIGHTNING FLASHES SEEN FROM SPACE (2000)
DJF
JJA
19
PEROXYACETYLNITRATE (PAN) AS RESERVOIR FOR
LONG-RANGE TRANSPORT OF NOx
20
NOx, HNO3, AND PAN OVER THE TROPICAL
PACIFICAircraft observations from PEM-Tropics
B, March-April 1999
Staudt et al. 2003
21
QUESTIONS
1. The prospect of hydrogen-fueled cars is
leading to study of the effect of increasing
atmospheric H2. Would you expect H2 to be
oxidized by OH? What would be the effect on the
oxidizing power of the atmosphere? 2. Loss
of NOx in the troposphere takes place by NO2OH,
same as in the stratosphere. What is the effect
of this reaction on tropospheric ozone?
22
QUESTION
1. How does the lifetime of NOx respond to a
change in the NOx concentration? If NOx
emissions were to double, would NOx
concentrations more than double or less than
double?