Topic 5: The Pb Biogeochemical Cycle - PowerPoint PPT Presentation
1 / 7
Title: Topic 5: The Pb Biogeochemical Cycle
1
Topic 5 The Pb Biogeochemical Cycle
2
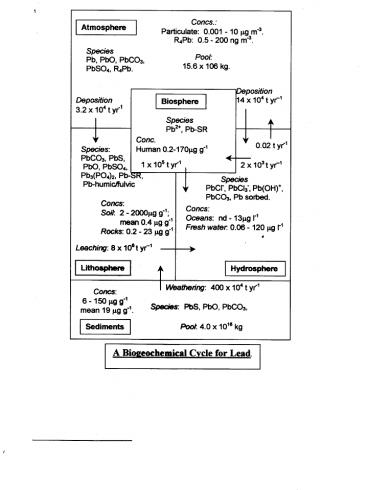
The Pb Biogeochemical Cycle
Consider the diagram of a cycle Pb is present
in all spheres but in low concentrations. It
is a trace element (lt1000ppm) in most matrices.
Fluxes (t yr-1 tonne yr-1 1000kg yr-1) are
due/result from mans activities the cycle is an
anthropogenically driven one. Mining flux
(1985) 4100 t yr-1 or about 10 times the natural
flux from lithosphere.
Atmospheric component almost solely from the
use of Pb in petrol as an octane booster (since
1950s), a practice which most countries have now
stopped (in Jamaica 0.75gPb dm-3 until 2000).
TR(atmosphere) 15.6 x 106/17.2 x 107 ? 1
month. Although 1 month is short compared to
atmospheric global circulation times (about 3
months) the Pb in Greenland ice data clearly show
the global impact of the use of Pb in petrol and
the importance of the atmospheric transport.
80 of the deposition is to the sea. The
variation in the Pb with depth in the oceans is
significantly different from those of the
nutrients. The Pb profile shows that while
biological uptake may be occurring the flux from
the atmosphere far exceeds the uptake and that
the depostion has yet to have an effect on the
deep oceans. Compare that with Cd, a toxic trace
metal, that behaves like a nutrient. Pburban
atmos. ? 0.1 -50 ng m-3. Pbrural atmos. ? 0.01
- 1.4 ng m-3. Pb pollution is predominantly an
urban issue.
Pb - 1
3
The Pb Biogeochemical Cycle cont.
The Biological Functions There are no known
essential biological functions. Most plants can
tolerate up to 10ppm (dry weight dw) and grow in
contaminated soils and aquifers. Many plants
accumulate Pb up to concentrations of 15000ppm
(dw) without obvious effects (lettuces).
Minimal uptake from soils, probably due to low
availability at normal pHs liming very
effective limiting availability. Uptake from
atmospheric sources can be readily adsorbed
through foliage (possibly remaining as
particulates in pores).
Pb is a serious toxin to humans. bones - 4 -
30ppm liver - 3 - 12ppm hair 3 - 70 ppm
nails - 14 - 170 ppm Take in about 200?g
day-1, 80 from food, 20 from atmosphere (50 in
the fine particulates). 10 of the intake is
adsorbed by the body of which 90 substitutes for
Ca ionic radii Pb2 98pm (CN 4), 119pm (CN 6)
Ca2 100pm (CN 6)
blood Pb concentrations of 4 - 50 ?g dl-1 in
adults and 10 - 20 ?g dl-1 in children known to
be toxic. Tolerances very variable, children
particularly susceptible to poisoning.
Physiological effects - nausea, vomiting,
abdominal pains, anemia, insomnia, mood
changes. - reduces fertility and can lead to
birth defects. - affects biosynthesis of haem,
inhibits Fe uptake, affects Cu and Zn enzymes.
Neurological effects - negatively affects IQ,
increases restlessness, limits attention spans.
Pb - 2
4
The Pb Biogeochemical Cycle cont.
Sources and Uses of Lead. Principal minerals
Galena PbS Ksp 3.2 x 10-28 used for
extraction of Pb. Cerussite PbCO3 Ksp 1.5 x
10-13 Anglesite PbSO4 Ksp 1.9 x
10-8 secondary minerals formed by the
weathering of galena. in Sphalerite (ZnS),
Chalcopyrite(CuFeS2), isomorphously for
K (IR 138pm), Sr2 (IR 118pm), Ba2 (IR
135pm), Ca2 (IR 100pm) Pb2 IR 118pm (CN 6)
98pm (CN 4). 0 - 50 ppm in most rocks
800 - 1000 ppm in Mn nodules 2 - 370 ppm
in coal Jamaican soils 6 - 897 ppm (mean
46.5ppm, n 203 95 lt 90ppm). Extraction 1.
Roasting (600C) 2PbS 3O2 ? 2PbO 2SO2. 2.
Smelting (400C) PbO CO ? Pb CO2. 3.
Purification slow cooling Cu (MP 1083C) floats
on the melt Zn (MP 420C) adsorbs Au, Ag and
crystallizes from the melt Sn, As, Sb all remain
as oxides Pb crystallizes at 327C. Uses 60 (4
x 106 tonnes yr-1) in Pb acid batteries - Pb
anode and a paste of oxides of Pb(IV) on a Pb
(91)/Sb (9) alloy grid cathode. Anode Pb
SO42- ? PbSO4 2e-. Cathode PbO2 4H
SO42- ? PbSO4 2H2O - 2e-. Net reaction Pb
PbO2 4H SO42- ? 2PbSO4 2H2O 2.1
volts 20 in the production of metal products
(solder, ammunition (20-25). 13 as pigments
red lead - Pb3O4 (rust resistant paint) white
lead 2PbCO3Pb(OH)2 (rust resistant paint)
yellow - PbCrO4 (road markings). Uses reducing
due to toxicities. 4 as R4Pb additive to petrol
(R ethyl and methyl). Acts as a free radical
trap. 1g additive dm-3 initially used. (63 PbR4,
26 BrCH2CH2Br, 9 ClCH2CH2Cl, 2 dye) PbR4
14O2 ? PbO2 8CO2 10H2O. PbO2
RCH2. ? PbO RCH2O. RCH2O. ( O2) ? RCHO.
PbO (RCl, RBr) ? PbCl2, PbBr2, PbClBr. 3
in glass, crystal, glazes, etc.
Pb - 3
5
(No Transcript)
6
The Pb Biogeochemical Cycle Environmental
Reactions cont.
In fresh waters when PbCO3 controls the Pb2
HNTA2- and HCO3- will be the dominant dissolved
free ligand and DIC species respectively.
PbCO3 ? Pb2 CO32- Ksp
1.48 x 10-13 CO32- H3O ? HCO3-
H2O Kb 2.13 x 1010 HNTA2- H2O ?
NTA3- H3O Ka3 5.25 x 10-11
Pb2 NTA3- ? PbNTA- Kf 2.45 x 1011 ?
PbCO3 HNTA2- ? PbNTA- HCO3 K Ksp x Kb x
Ka3 x Kf. K/HCO3- PbNTA-/HNTA2- 42 when
an alkalinity of 1?M. Thus if there is any Pb2
in solution it will be complexed by any available
ligand. If the total concentration of ligand (
HNTA2- PbNTA-) is 1mM then PbNTA-/42
HNTA2- 1/42PbNTA- PbNTA- 1 x 10-3
? PbNTA- 1mM. I.e the solubility
of Pb(II) has been enhanced from 0.016mM to 1mM,
a factor of over 60.
However Pb(II) will compete for the ligand with
other metals present, particularly Ca(II) (total
concentration 1mM). At environmental pHs the
ligand will be equally divided between Pb(II) and
Ca(II). The major ligands will be fulvic
acids. Adsorption to particles has not been
considered that may decrease the dissolved phase
concentrations, depending on the magnitudes of
the equilibrium constants. Modelling is a major
research area.
Pb - 5
7
Analytical Methods for Pb.
Pb is usually present in trace amounts and thus
samples are very easy to contaminate. Follow
procedures, prepare many blanks, use SRMs, take
and analyse many duplicates, be involved in
inter- and intra- laboratory comparisons. It is
generally accepted that pre 1960s data are
unreliable and that much of the current data
should be considered with caution. a) In the
atmosphere present as particulates. Collect
settling particles in settling jars. Suspended
particles by pumping (PM10, PM2.5) at known
volumes per hour. Digest in acid (HCLO4,
HNO3/HCL HF) and analyse by GFAAS, AAS, ICP-AES.
In solid phase or on filter - XRF. b) In
sediments/soils - as for particulates. c) In
water - very low concentrations GFAAS, ICP-AES.
Speciate by pretreatments using exchange columns
or electrochemical methods - operationally
defined and difficult. d) Other matrices - hair,
nails, blood, biological tissues - sample
pretreatment required. Sampling and storage.
avoid contamination - sampling instruments,
bottles (HNO3 cleaned), reagents (super pure
acids), clean rooms (filtered air and limited
access). 70 can adsorb to container surfaces
in 4 days at pH 6 5 in 4 days at pH 2 - acidify
samples if they must be stored. in-homogeneity
in soils and sediments, un-equal uptake by parts
of plants, animals (hair and nail length, age of
plants, organs of animals).
Some Environmental Problems Battery recycling
shops. Shopworker. Shop. Worker.
Control. House dust (ppm) 10,000
3000 1700 500 General yard soil
3000 3000 100
100 Soil near contamination 50,000
6000 100 100 blood (WHO ,
40 ?g dl-1 in adults) 0 - 5 years old
(?g dl-1) 110
55 14 14 6 -
11 years old
56 54 23
12 over 12
32 18
10 7 Figueroa et al.,
1987, MOH.
Pb - 6