The First Law of Thermodynamics
1 / 13
Title:
The First Law of Thermodynamics
Description:
For combustion reaction, use adiabatic flame calorimeter (left) measure temp ... During adiabatic change, temperature decrease is expected because work is done ... –
Number of Views:27
Avg rating:3.0/5.0
Title: The First Law of Thermodynamics
1
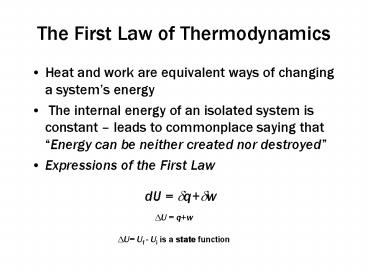
The First Law of Thermodynamics
- Heat and work are equivalent ways of changing a
systems energy - The internal energy of an isolated system is
constant leads to commonplace saying that
Energy can be neither created nor destroyed - Expressions of the First Law
- dU dqdw
DU qw DU Uf - Ui is a state function
2
Heat transactions
- At constant volume, system only capable of PdV
work - dU dq DUqV
- So changes in the internal energy can be
measured by measuring heat produced - Calorimetry is the science of measuring heat
produced in chemical reactions and phase
transitions - -- bomb calorimetry
- -- isothermal titration calorimetry (ITC)
- -- differential scanning calorimetry (DSC)
3
Heat Capacity at Constant Volume
Internal energy of a substance increases as
temperature is raised
For a constant volume process
Molar heat capacity CV,m Specific heat Cv/mass
4
Dont Lose Sight of the Importance of Partial
Derivatives
In general, U depends on V and TWe will show
that
This ability to expand state functions in terms
of their partial derivatives is an essential
concept to understand in t thermodynamics
5
Some important relationships (at constant volume)
- dU CVdT
- DU CVDT
- qV CVDT
- To measure CV ? supply a known quantity of heat
(electrically) and measure DT - Suppose volume is not constant
- Most chemical reactions are carried out at
constant pressure - Would be useful to have an expression for qp
6
Enthalpy
Heat supplied at constant pressure is equal to
the change in the enthalpy H of the system
Defining H UpV ? dH dq (for constant
pressure, with no additional work) DH
qp H is also a state function
When system is free to change its volume at
constant pressure, some of the energy supplied as
heat may escape back to the surroundings as work
? dU lt dQ
7
Another Approach to Enthalpy
H plays the same role in a constant-pressure
process that U plays in a constant-volume
process
8
Measurement of Enthalpy Change
Monitor temperature change that accompanies
chemical change at constant pressure ? isobaric
calorimeter For combustion reaction, use
adiabatic flame calorimeter (left) ? measure temp
change when certain amount of substance burns in
O2
9
Heat Capacity at Constant Pressure
Enthalpy of a substance increases as temperature
is raised
For a constant pressure process
Molar heat capacity Cp,m
dH CpdT DH CpDT qp CpDT
Cp,mgt CV,m
10
Relations for an Ideal Gas
- We will derive that Cp-Cv nR
It usually holds in condensed phases that DH
DU, except at high pressures
11
Adiabatic Process No energy transferred as heat
During adiabatic change, temperature decrease is
expected because work is done and the internal
energy falls In molecular terms, KE of molecules
falls, so average molecular speed decreases and
hence T falls Decompose changes in Internal Energy
12
Adiabats and Isotherms
P declines more steeply for an adiabat than for
an isotherm because T decreases in adiabat
13
Coming on Monday
- Reaction Enthalpies