N - PowerPoint PPT Presentation
Title: N
1
(No Transcript)
2
(No Transcript)
3
(No Transcript)
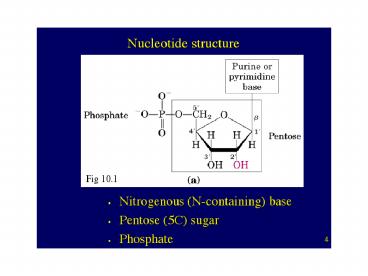
4
(No Transcript)
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
Nucleotides play key roles in many, many cellular
processes
1. Activated precursors of RNA and DNA 2.
Adenine nucleotides are components of three major
co-enzymes, NAD, FAD, and CoA 3. Nucleotide
derivatives are activated intermediates in
biosynthetic processes (UDP-glucose, SAM) 4.
Serve as metabolic regulators (for example cAMP
and the activation of cell signaling. 5.
Serve as major currency of energy in all cells
(ATP and GTP). 6. Many metabolic diseases
have their etiology in nucleotide
metabolism.
14
Purine metabolism (Overview) 1.
Nomenclature/nucleotide structure 2. De novo
synthesis pathways 3. Re-utilization
pathways 4. Metabolic diseases of purine
Metabolism(Gout, Lesch-Nyham, SCID)
15
(No Transcript)
16
Why go through to the trouble to convert Uracil
to Thymine?
reduced
oxidized
NADPH
Dihydrofolate reductase
Serine transhydroxymethylase
NADP
17
The nomenclature of purines and pyrimidines
depends on their linkage to a pentose
Cytosine
Cytidine
Cytidine Monophosphate
Nucleoside Base
Base
Nucleotide Base (P04 ester)
when the base is purine, then the nucleoside
ends in OSINE (AdenOSINE, GuanOSINE, InOSINE)
when the base is pyrimidine, then the nucleoside
ends in IDINE (UrIDINE, CytIDINE, ThymIDINE)
18
The active forms of nucleotides in biosynthesis
and energy conversions are di and triphosphates
Nucleoside Monophosphate Kinase
Nucleoside Diphosphate Kinase
19
RIBONUCLEOTIDE REDUCTASE 1. Complex enzymatic
reaction whereby electrons are transferred from
NADPH through a series of sufhydryl groups at
the catalytic site of Ribonucleotide Reductase.
2. Active site of RR contains thioredoxin, a 12
kD protein with two exposed cysteines, which
become oxidized. 3. This ultimately allows for
the reduction of ribose. REGULATION 1. Based on
the response to cellular need for dATPs.
NADPH
NADP
dATP is general inhibitor ATP is a general
activator
20
Nucleotides are linked by 5 to 3 phosphodiester
bonds to generate DNA and RNA
21
Structures of Common Purine Bases.
H 6 oxy purine X 2,6 dioxy purine
A 6 amino purine G 2 amino, 6-oxy purine
22
Structures of Common Purine Bases.
H 6 oxy purine X 2,6 dioxy purine
A 6 amino purine G 2 amino, 6-oxy purine
23
Structures of Common Purine Bases.
(N source)
Aspartate
(N source)
Glutamine
The common mechanistic them for the conversion of
A and G is the conversion of a carbonyl oxygen
to an amino group
24
There are two basic mechanisms to generate
purines and pyrimidines
1. DE NOVO BIOSYNTHETIC PATHWAYS (building the
bases from simple building blocks)
2. SALVAGE PATHWAYS (the reutilization of bases
from dietary or catabolic sources)
25
The biosynthesis of purine (A and G) begins with
the synthesis of the ribose-phosphate
Pentose phosphate pathway
Ribose phosphate pyrophosphoKINASE
26
The major regulatory step in purine biosynthesis
is the conversion of PRPP to 5-Phosphoribosyl-1-a
mine
PPi
Amidophosphoribosyl transferase
Amidophosphoribosyl transferase is a important
regulatory enzyme in purine biosynthesis. It
is strongly inhibited by the end products IMP,
AMP, and GMP. This type of inhibition is called
FEEDBACK INHIBITION.
27
Several amino acids are utilized in purine
biosynthesis,
IMP is the precursor for both AMP and GMP, the
base is also called hypoxanthine
28
(No Transcript)
29
Structures of Common Purine Bases.
(N source)
Aspartate
(N source)
Glutamine
The common mechanistic them for the conversion of
A and G is the conversion of a carbonyl oxygen
to an amino group
30
Purineswhere do the atoms come from?
Purine intermediates include 1. Glycine 2. 1 C
units of 5,10 mTHF 3. Glutamine 4. Asparate
31
The regulation of purine biosynthesis is a
classic example of negative feedback
Inhibited by AMP
AMP
Phosphoribosyl amine
IMP
GMP
Inhibited by IMP, AMP, and GMP
Inhibited by GMP
32
(No Transcript)
33
Nucleotidase
Phosphorylase
Cytosine
Cytidine
Cytidine Monophosphate
Nucleoside Base
Base
Nucleotide Base (P04 ester)
34
Salvage pathways for the re-utilization of
purines There are 2 salvage enzymes with
different specificities 1. Adenine
phosphoribosyl transferase 2. Hypoxanthine-guanine
phosphoribosyl transferase
PPi
Guanine
A-PRT
HG-PRT
PRPP Guanine
Guanylate
35
What happens in gout?
Inhibited by AMP
AMP
Ribose 5-phosphate
PRPP
Phosphoribosyl amine
IMP
GMP
Inhibited by IMP, AMP, and GMP
Inhibited by GMP
1. Negative regulation of PRPP Synthatase PRPP
Amidotransferase is lost 2. PRPP levels are
increased because of defects in salvage
pathways Therefore, there is net increase in
biosynthetic/degradation pathways!!
36
The Gout James Gilray, 1799.
37
By Royal Authority by George Cruickshank. 19th
century.
38
David Wells New York Yankees
39
Purines in humans are degraded to Urate
Important points
1. Nucleotides are constantly undergoing
turnover! 2. There are many enzymes involved
Nucleotidases Nucleoside phosphorylases
Deaminases Xanthine oxidases 3. the final
common intermediate in humans is Urate,
which is excreted. 4. there are several
metabolic disorders resulting from defects
in purine catabolism.
40
GOUT (Gouty Arthritis) A defect of purine
metabolism
Allopurinol a. decrease urate b. increase
xanthine hypoxanthine c. decrease PRPP
41
SCID-Severe Combined Immunodeficiency Syndrome
Autosomal recessive disorder Mutations in
ADA Infants subject to bacterial, candidiasis,
viral, protazoal infections Both T and B cells
are significantly Reduced (dATP is
toxic) 1995-AdV expressing ADA was sucessfullly
employed as gene therapy strategy
42
Disorders of Purine Metabolism
Disorder Defect Comments
Gout PRPP synthase/
Hyperuricemia HGPRT
Lesch Nyhan lack of HGPRT Hyperuricemia
syndrome
SCID ADA high levels of dAMP
von Gierkes disease glucose -6-PTPase Hyperuricem
ia
43
Structure of Pyrimidines
C 2 oxy, 4 amino pyrimidine T 2,4 dioxy
5-methyl pyrmidine
U 2,4 dioxy pyrimidine O 2,4 dioxy 6 carboxy
pyrimidine
44
Pyrimidines where do the atoms come from?
45
Pyrimidine biosynthesis
(occurs in cytosol)
Pyrimidine biosynthesis begins with the assembly
of the ring, then linked To ribose phosphate.
Precursors are Glutamine (NH2), Bicarbonate (C)
, and ATP (PO4). Q. Why is it advantageous to
generate carbamoyl phosphate in the cytosol
rather than the mitochondria?
46
ATCase is the committed step in pyrimidine
biosynthesis
47
The second phase of pyrimidine biosynthesis
Note, in pyrimidine biosynthesis, the addition of
ribose phosphate moiety occurs late in the
pathway, via its addition of Orotate.
48
(No Transcript)
49
ATCase is feedback inhibited by the end-products
of pyrimidine biosynthesis
C02 Glutamine ATP
Carbonyl Phosphate
Inhibited by CTP
Carbonyl Asparate
UMP
UTP
CTP
50
Why go through to the trouble to convert Uracil
to Thymine?
reduced
oxidized
NADPH
Dihydrofolate reductase
Serine transhydroxymethylase
NADP
51
(No Transcript)
52
Common chemotherapeutic drugs act at the level
of dTMP synthesis.
53
AZT is used to inhibit HIV reverse transcriptase
(RNA-dependent DNA pol)
3 azido-23 dideoxythymine (AZT)
This class of compounds (chemotherapeutics, viral
inhibitors, etc are called nucleoside analogs.
54
Common side effects of DNA inhibitor
chemotherapeutics Diarrhea Skin and eye
sensitivity to sunlight Abnormal liver function
tests Hair loss Immuno-suppression
Skin rashes Fatigue Headache, backache,
Spinal cord irritation Peripheral neuropathies
55
Summary 1. Recognize basic structures of
purines and pyrimidines 2. Key regulatory
enzymes and feedback networks 3. Targets for
clinical interventions