Chapter 6' Intro to Spectroscopic Methods - PowerPoint PPT Presentation
1 / 30
Title:
Chapter 6' Intro to Spectroscopic Methods
Description:
Orbital picture: hn. e- spins are paired. orbital NRG diagram ... core e- absorb X-ray (high NRG) valence e- absorb UV-vis (not as high NRG) ... – PowerPoint PPT presentation
Number of Views:55
Avg rating:3.0/5.0
Title: Chapter 6' Intro to Spectroscopic Methods
1
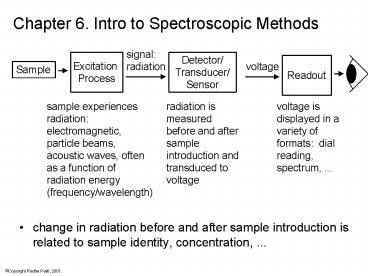
Chapter 6. Intro to Spectroscopic Methods
signal radiation
Detector/ Transducer/ Sensor
Excitation Process
Readout
voltage
Sample
sample experiences radiation electromagnetic,
particle beams, acoustic waves, often as a
function of radiation energy (frequency/wavelength
)
radiation is measured before and after sample
introduction and transduced to voltage
voltage is displayed in a variety of formats
dial reading, spectrum, ...
- change in radiation before and after sample
introduction is related to sample identity,
concentration, ...
2
A. Electromagnetic radiation (EMR) - properties
- 1. Wave properties
- Why the term electromagnetic radiation?
- speed, amplitude, wavelength, frequency
- higher NRG vs. lower NRG
- monochromatic
- polychromatic
3
2. Principle of superposition of waves
- if two or more waves overlap in space,
- electromagnetic disturbance at a given point
equals the sum of the e-mag disturbances from
each wave at that point. - in-phase vs. out-of-phase
- constructive interference resultant wave has
max amplitude - destructive interference resultant wave has min
amplitude
4
B. The anatomy of EMR absorption
- atoms, ions and molecules can absorb EMR in
quantized increments - how do these oscillating fields interact with
atoms/molecules/ions? - electronic transitions
- vibrational transitions
- rotational transitions
5
1. Basics of NRG level diagram
Consider a simple two-level NRG diagram of
molecule X. These are not necessarily electronic
NRG levels! They could be electronic,
vibrational or rotational NRG levels.
excited state
upon absorbing a photon (hn)
ground state
molecule X in ground state
molecule X in excited state
what is the fate of excited state?
6
2. Fate of excited state
Excited state has some lifetime (picosecond to
even seconds long - long-lived excited state)
molecule X in excited state
Where does NRG of excited state go? (after all,
excited state was generated by absorption of a
photon)
7
3. NRG from excited state relaxation?
- Emission of a photon of NRG less than or equal to
that absorbed (why?) - non-radiative emission
- (solvent picks up NRG in its vibrations, or
thermal emission)
8
4. NRG-level diagram, Part 2
Lets make the previous NRG-level diagram more
specific - an electronic transition
9
5. Electron spins
- Electronic state with paired e- spins is called
singlet - singlet can be transformed into triplet
- triplet electronic state in which e- spins are
unpaired, or each e- of a pair has same spin - Orbital diagrams
10
6. NRG level diagram, part 3
- Each electronic energy level has several
sublevels vibrational levels.
e2 2nd excited vibrational state of E1
E1
e1 1st excited vibrational state of E1
e0 ground vibrational state of E1
e2 2nd excited vibrational state of E0
E0
e1 1st excited vibrational state of E0
e0 ground vibrational state of E0
Note each vib state has rotational sublevels.
11
7. Lots of possible transitions!
E1
Lets assume that molecule X begins in the E0 e0
state. What transitions can we draw?
E0
each transition has its own propensity to absorb
(absorptivity, cross-section)
12
8. Why are these hn s interesting?
- Transitions between E0 and E1 (and higher) tend
to be in the uv/visible range. - These tell us about electronic structure (for
example, energy of HOMO-LUMO gap, which orbitals
e- move in and out of). - Transitions between e0 and e1 (and higher) tend
to be in the IR range. - They tell us about
- Lets put NRGs in order from lowest NRG to
highest NRG.
13
C. Absorption by types of chemical species
- 1. molecules/polyatomic ions/complexes
- lots of possible transitions
- How does this look as a spectrum?
high
NRG absorbed
low
high
low
Frequency of irradiating NRG, or ...
14
2. Atoms/monoatomic ions
- EMR absorbed by electron jumping to higher NRG
level - only electronic transitions
- no vibrational or rotational - why not?
sharp peaks - why? core e- absorb X-ray (high
NRG) valence e- absorb UV-vis (not as high
NRG) general rule the harder it is to move the
e- or make the transition, the higher the NRG
necessary to do it.
NRG absorbed
Frequency of NRG
15
3. Transmittance and absorbance
Po
Po
detector
Po initial radiant power
P radiant power
Po
P
A - log T
16
D. Emission of radiation
- After a chemical species absorbs NRG, that
species is in an excited state. - absorbed NRG can be in the form of EMR, or
current, or electron beam, or flame - Excited state relaxes back to ground state by
nonradiative and radiative pathways. - Radiative relaxation - examples
- line spectrum - Hg
- band spectrum - fluorescent lights
- continuum spectrum (blackbody radiation)
17
Chapter 7. Components of Optical Instruments
signal radiation
Detector/ Transducer/ Sensor
Excitation Process
Readout
voltage
Sample
sample is exposed to NRG from a radiation SOURCE
the sample absorbs NRG (and sometimes emits NRG
too)
a WAVELENGTH SELECTOR separates NRG by wavelength
(l) ...and lets NRG at each l impinge on a
TRANSDUCER that converts radiation from sample
into voltage
We will build a prototypical optical instrument
as we study each component part.
18
A. Sources
- 1. continuum - emits a wide continuous range of
l at constant intensity - tungsten lamp - emits uv
- deuterium lamp -emits visible
- hot inert solids - emits IR
- 2. line - emits a few l
- Hg lamp
- hollow cathode lamp
- 3. laser - learn mechanism from text
- common lasers include HeNe (632.8 nm), NdYAG
(532 nm), diode lasers (variable l)
19
4. Our optical instrument
radiation source
sample
What are the important properties of the
radiation passing through sample? How do we
measure those properties?
sample can be placed in radiation beam in many
ways
20
B. Wavelength selectors
- 1. Concepts and terms
- what is the general purpose of a wavelength
selector? - How well does a wavelength isolate a single
wavelength? - effective bandwidth
21
2. Filters
- colored glass filters
- band pass
- long pass
- short pass
- interference filters - all wavelengths experience
destructive interference except wavelength of
interest
22
3. Prism monochromator
- What does a prism do to light passing through it?
23
4. Grating monochromator
- a. What is a grating?
- grating a hard, optically flat, polished
surface with closely grooves (or blazes) cut into
it
transmission grating - light passes through
grating reflection grating - light reflects off
surfaces (we will emphasize reflection
grating)
24
B. What does a grating do?
- grating sends light of only one l at a particular
angle (where detector is waiting). This happens
through constructive interference of that l.
detector
radiation source
Light at l 400 nm
45
Only 400 nm undergoes constructive interference
sample
In order to record full spectrum, either detector
or grating must move.
25
c. How does a grating work? (diffraction)
- diffraction - when light passes through a small
aperture or a slit of width comparable to that of
lights wavelength, light appears outside region
of geometrical shadow - light is said to bend around sharp boundary
- Aperture/slit is considered a point source of
light. - Grating simply involves many small point sources.
A wavelength (ie. 400 nm) only constructively
interferes at a certain angle (45)
26
5. Monochromator slit width
- Light from sample passes through an entrance slit
before reaching the grating, and exits through an
exit slit before reaching the detector. - Width of slits strongly affects wavelength
resolution of light. - The smaller the slit, the better resolved the
wavelength.
27
6. Our optical instrument, part 2
radiation source
sample
What should we do with wavelength-separated light?
28
C. Transducers
- The game of all these transducers is usually to
produce an electron-hole pair by impinging a
photon onto material. - hole lack of e- h
- 1. photoresistors - circuit elements whose
resistance changes with exposure to light - 2. photovoltaic cells
- 3. phototubes
29
3. photomultiplier tubes (PMTs)
- 4. photodiodes/diode arrays Si diode biased so
that conductivity zero. - A photon impinging on diode makes an e- -hole
pair. - An array of diodes can measure current of each
diode separately. - 5. Charge injection device(CID) /Charge-coupled
device (CCD) - semiconductor diodes in a 2-D
array - pixels
30
6. Our optical instrument, part 3
radiation source
entrance slit
sample
grating
exit slit
Transducer - produces voltage which goes to
readout