Quantum Theory of Atoms - PowerPoint PPT Presentation
Title:
Quantum Theory of Atoms
Description:
The Bohr theory of Hydrogen(1913) cannot be extended to other atoms with more than one electron ... are the same as in the Bohr theory En = -Z2 (13.6 eV)/n2 ... – PowerPoint PPT presentation
Number of Views:24
Avg rating:3.0/5.0
Title: Quantum Theory of Atoms
1
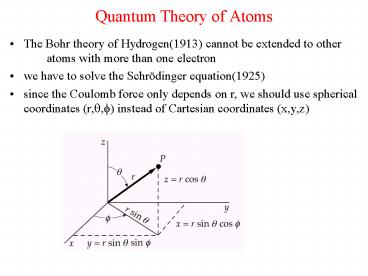
Quantum Theory of Atoms
- The Bohr theory of Hydrogen(1913) cannot be
extended to other atoms with more than one
electron - we have to solve the Schrödinger equation(1925)
- since the Coulomb force only depends on r, we
should use spherical coordinates (r,?,?) instead
of Cartesian coordinates (x,y,z)
2
Quantum Theory of Atoms
- For a particle in a cubic or rectangular box,
Cartesian coordinates are more appropriate and
there are three quantum numbers (n1,n2,n3) needed
to label a quantum state - similarly in spherical coordinates, there are
also three quantum numbers needed - n 1, 2, 3, ..
- l 0, 1, 2, ,n-1 gt n values of l
for a given value of n - m -l,(-l1),0,1,2,,l gt 2l1 values of m
for a given l - n is the principal quantum number and is
associated with the distance r of an electron
from the nucleus - l is the orbital quantum number and the angular
momentum of the electron is given by
Ll(l1)1/2 h - m is the magnetic quantum number and the
component of the angular momentum along the
z-axis is Lz m h
3
Quantum Theory of Atoms
- The fact that both l and m are restricted
to certain values is due to boundary
conditions - in the figure, l2 is shown
- hence L(2(21))1/2 h h(6)1/2
- and m -2, -1, 0, 1, 2
- the Schrödinger equation can be
solved exactly for hydrogen - the energies are the same as in the Bohr
theory En -Z2 (13.6 eV)/n2 - they do not depend on the value of l and m
- this is a special property of an inverse-square
law force
4
- The lowest energy has n1 gt l0 and m0
- the second lowest energy has n2 gt l0,
m0 l1, m-1,0,1 - hence 4 states!
- Notation l0 S-state
- l1 P-state
- l2 D-state
- l3 F-state
- l4 G-state
5
- When a photon is emitted or absorbed we must
have ?m 0 or 1 ?l 1 - conservation of angular momentum and conservation
of energy
6
Wave Functions
- We denote the solutions of the SE as ?nlm
(r,?,?) - the probability of finding the electron at some
position (r,?,?) is P (r,?,?) (?nlm)2 dV where
dV is the volume element - in spherical coordinates dVr2drsin?d?d?
7
Wave Functions
- the ground state wave function is Cexp(-Zr/a0)
where C is determined from normalization
- Hence for the ground state, the probability
density ?1002 is independent of ? and ? and is
maximum at the origin
8
Wave Functions
- What is the probability of finding the electron
between r and rdr? - in other words, in a spherical shell of thickness
dr - volume of shell is 4?r2 dr (area x
thickness) - P(r)dr (4?r2 ?2)dr P(r) is the radial
probability density
9
Wave Functions
- Maximum is at r a0/Z a0 for Hydrogen (first
Bohr orbit) - not a well defined orbit but rather a cloud
10
Probability density