E at Nonstandard Conditions - PowerPoint PPT Presentation
1 / 27
Title:
E at Nonstandard Conditions
Description:
1 2006 Brooks/Cole - Thomson. E at Nonstandard Conditions. The ... 2 NH4 2e ... magnetic props. of matter. electromagnetic induction. benzene and other ... – PowerPoint PPT presentation
Number of Views:36
Avg rating:3.0/5.0
Title: E at Nonstandard Conditions
1
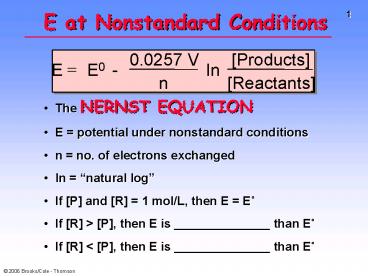
E at Nonstandard Conditions
- The NERNST EQUATION
- E potential under nonstandard conditions
- n no. of electrons exchanged
- ln natural log
- If P and R 1 mol/L, then E E
- If R gt P, then E is ______________ than E
- If R lt P, then E is ______________ than E
2
BATTERIESPrimary, Secondary, and Fuel Cells
3
Dry Cell Battery
Primary battery uses redox reactions that
cannot be restored by recharge.
- Anode (-)
- Zn ---gt Zn2 2e-
- Cathode ()
- 2 NH4 2e- ---gt 2 NH3 H2
4
Alkaline Battery
- Nearly same reactions as in common dry cell, but
under basic conditions.
Anode (-) Zn 2 OH- ---gt ZnO H2O
2e- Cathode () 2 MnO2 H2O 2e- ---gt
Mn2O3 2 OH-
5
Lead Storage Battery
- Secondary battery
- Uses redox reactions that can be reversed.
- Can be restored by recharging
6
Lead Storage Battery
- Anode (-) Eo 0.36 V
- Pb HSO4- ---gt PbSO4 H 2e-
- Cathode () Eo 1.68 V
- PbO2 HSO4- 3 H 2e- ---gt PbSO4 2
H2O
7
Ni-Cad Battery
- Anode (-)
- Cd 2 OH- ---gt Cd(OH)2 2e-
- Cathode ()
- NiO(OH) H2O e- ---gt Ni(OH)2 OH-
8
Electrolysis
- Using electrical energy to produce chemical
change. - Sn2(aq) 2 Cl-(aq) ---gt Sn(s) Cl2(g)
9
Electrolysis of Aqueous NaOH
Electric Energy ----gt Chemical Change
- Anode ()
- 4 OH- ---gt O2(g) 2 H2O 4e-
- Cathode (-)
- 4 H2O 4e- ---gt 2 H2 4 OH-
- Eo for cell -1.23 V
Anode
Cathode
10
Electrolysis Electric Energy ---gt Chemical
Change
Electrolysis of molten NaCl. Here a battery
pumps electrons from Cl- to Na. NOTE
Polarity of electrodes is reversed from batteries.
11
Electrolysis of Molten NaCl
Figure 20.18
12
Electrolysis of Molten NaCl
- Anode ()
- 2 Cl- ---gt Cl2(g) 2e-
- Cathode (-)
- Na e- ---gt Na
Eo for cell (in water) Ec - Ea - 2.71 V
(1.36 V) - 4.07 V (in water) External
energy needed because Eo is (-).
13
Electrolysis of Aqueous NaCl
- Anode ()
- 2 Cl- ---gt
- Cl2(g) 2e-
- Cathode (-)
- 2 H2O 2e- ---gt
- H2 2 OH-
- Eo for cell -2.19 V
- Note that H2O is more easily reduced than
Na.
Also, Cl- is oxidized in preference to H2O
because of kinetics.
14
Electrolysis of Aqueous NaCl
- Cells like these are the source of NaOH and Cl2.
- In 1995 25.1 x 109 lb Cl2 and 26.1 x 109 lb NaOH
Also the source of NaOCl for use in bleach.
15
Electrolysis of Aqueous NaI
- Anode () 2 I- ---gt I2(g) 2e-
- Cathode (-) 2 H2O 2e- ---gt H2 2 OH-
- Eo for cell -1.36 V
16
Electrolysis of Aqueous CuCl2
- Anode ()
- 2 Cl- ---gt Cl2(g) 2e-
- Cathode (-)
- Cu2 2e- ---gt Cu
- Eo for cell -1.02 V
- Note that Cu is more easily reduced than either
H2O or Na.
17
Electrolytic Refining of Copper
Figure 22.11
Impure copper is oxidized to Cu2 at the anode.
The aqueous Cu2 ions are reduced to Cu metal at
the cathode.
18
Producing Aluminum
- 2 Al2O3 3 C ---gt 4 Al 3 CO2
Charles Hall (1863-1914) developed electrolysis
process. Founded Alcoa.
19
Michael Faraday1791-1867
- Originated the terms anode, cathode, anion,
cation, electrode. - Discoverer of
- electrolysis
- magnetic props. of matter
- electromagnetic induction
- benzene and other organic chemicals
- Was a popular lecturer.
20
Eo and Thermodynamics
- Eo is related to ?Go, the free energy change for
the reaction. - ?G proportional to nE
- ?Go -nFEo
- where F Faraday constant 9.6485 x
104 J/Vmol of e- - (or 9.6485 x 104 coulombs/mol)
- and n is the number of moles of electrons
transferred
21
Eo and ?Go
- ?Go - n F Eo
- For a product-favored reaction
- Reactants ----gt Products
- ?Go lt 0 and so Eo gt 0
- Eo is positive
- For a reactant-favored reaction
- Reactants lt---- Products
- ?Go gt 0 and so Eo lt 0
- Eo is negative
22
Quantitative Aspects of Electrochemistry
- Consider electrolysis of aqueous silver ion.
- Ag (aq) e- ---gt Ag(s)
- 1 mol e- ---gt 1 mol Ag
- If we could measure the moles of e-, we could
know the quantity of Ag formed. - But how to measure moles of e-?
23
Quantitative Aspects of Electrochemistry
- But how is charge related to moles of electrons?
96,500 C/mol e- 1 Faraday
24
Quantitative Aspects of Electrochemistry
- 1.50 amps flow thru a Ag(aq) solution for 15.0
min. What mass of Ag metal is deposited? - Solution
- (a) Calc. charge
- Charge (C) current (A) x time (t)
- (1.5 amps)(15.0 min)(60 s/min) 1350 C
25
Quantitative Aspects of Electrochemistry
1.50 amps flow thru a Ag(aq) solution for 15.0
min. What mass of Ag metal is deposited?
- Solution
- (a) Charge 1350 C
- (b) Calculate moles of e- used
(c) Calc. quantity of Ag
26
Quantitative Aspects of Electrochemistry
- The anode reaction in a lead storage battery is
- Pb(s) HSO4-(aq) ---gt PbSO4(s) H(aq) 2e-
- If a battery delivers 1.50 amp, and you have 454
g of Pb, how long will the battery last? - Solution
- a) 454 g Pb 2.19 mol Pb
- b) Calculate moles of e-
c) Calculate charge 4.38 mol e- 96,500 C/mol
e- 423,000 C
27
Quantitative Aspects of Electrochemistry
- The anode reaction in a lead storage battery is
- Pb(s) HSO4-(aq) ---gt PbSO4(s) H(aq) 2e-
- If a battery delivers 1.50 amp, and you have 454
g of Pb, how long will the battery last? - Solution
- a) 454 g Pb 2.19 mol Pb
- b) Mol of e- 4.38 mol
- c) Charge 423,000 C
d) Calculate time
About 78 hours