Onset of cavitation bubble formation - PowerPoint PPT Presentation
1 / 31
Title: Onset of cavitation bubble formation
1
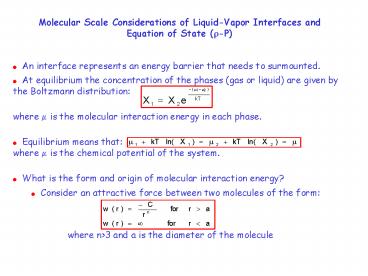
Molecular Scale Considerations of Liquid-Vapor
Interfaces and Equation of State (?-P)
- An interface represents an energy barrier that
needs to surmounted. - At equilibrium the concentration of the phases
(gas or liquid) are given by the Boltzmann
distribution - where ? is the molecular interaction energy in
each phase. - Equilibrium means thatwhere ? is the chemical
potential of the system. - What is the form and origin of molecular
interaction energy? - Consider an attractive force between two
molecules of the form
where ngt3 and a is the diameter of the molecule
2
Molecular Scale Considerations of Liquid-Vapor
Interfaces and Equation of State (?-P) -
continuation
- We calculate ?1 by summing pair potentials w(r)
over all space - where ? is the number density of the molecules in
space. - The effective density of non-ideal gas including
volume excluded B4?a3/3
- The chemical potential of a gas ? is thus
- Relating the pressure P to ? by
- Integrating to relate the pressure P to ? and
obtain the equation of state
3
van der Waals equation of state
Ideal gas law (EOS)
Non-ideal gas law (van der Waals
- a A/2 a term due to molecular attractive forces
atm L2 mol-2 - bB/22?a3/3 a term due to finite volume of
molecules L mol-1
4
CO2 P-V Space
- EOS represents a balance between attractive and
repulsive forces in a given background of kT
(disordering force). - Denser materials require larger kT due to
stronger intermolecular interactions
(vdW?polar?covalent). - Similarly liquid vs. gas (vapor)
5
Liquid-Vapor Coexistence CO2, P-V Space
Vapor Pressure
Vapor Molar Volume
Liquid Molar Volume
6
Maxwell Construction CO2, P-V Space
Maxwell Construction Area A Area B
Gives --vapor pressure--densities of
coexisting liquid and vapor
Vapor Pressure
B
Vapor Molar Volume
A
Liquid Molar Volume
Flat interfaces only!
7
van der Waals Non-QuantitativeWater, 298K P- r
Space
8
Including Directional H-BondsWater, 298K P- r
Space
9
EOS/van der Waals Summary
- van der Waals equation gives a simple qualitative
explanation of phase separation based on
molecular attraction and finite molecular size - Maxwell construction gives the vapor pressure and
the densities of coexisting liquid and gas at
equilibrium, FOR FLAT INTERFACES. - van der Waals equation fails to quantitatively
reproduce the EOS of water
10
Capillarity
Curved Liquid-Vapor Interfaces
- Curved liquid-vapor interfaces result in pressure
difference between the liquid and vapor phases
depending on the direction of curvature. - In porous media, curved interface are often
anchored on solid surface at a certain contact
angle. - Capillarity give rise to an array of important
phenomenon in unsaturated porous media.
11
The Young-Laplace Equation
Neglecting terms of order dr2 and higher
12
Cavitation (spontaneous formation of a bubble)
alternative derivation of the Young-Laplace
Equation
P
13
Capillary rise in a cylindrical tube
Vertical force balance
Upward force (capillary pull)
Downward force (weight of water)
14
Surface tension values (Adamson, 1990)
15
Measurement of surface tension The ring method
(du Nouy 1919)
- The method is simple and measures the detachment
force(the surface tension multiplied by the
periphery 22?R) - Often using a platinum ring flamed before use and
torsion wire is used for force measurements. - Errors due to internal and planar curvatures
require some modifications.
Wilhelmy slide (1863)
- p is the perimeter of a thin slide no
corrections are needed!
16
Measurement of surface tension The maximum
bubble pressure method
- A bubble of inert gas is slowly blown into the
liquid. - The bubble shape (curvature) goes through a
minimum (maximum pressure in U-tube) when bubble
radius is equal the tube radius. - We then use the Young-Laplace equation with r and
?P known.
17
Measurement of surface tension Drop weight
method
- The drop weight is supported by surface tension
around the tip. - The actual detachment pattern occurs below the
tip and complicates the calculations. - Sophisticated computer algorithms are combined
with image analysis methods to solve for the drop
shape with the surface tension as the matching
parameter
18
Capillarity and Porous Media
- Curved liquid-vapor interfaces are formed in
unsaturated porous media.
19
The Bundle of Cylindrical Capillaries Model
- The conceptual step
- Liquid in partially saturated porous media is
considered to be held in a pore space resembling
a bundle of cylindrical capillaries. - Cut a random-rejoin models
- Limitations
- No dual occupancy
- Ignoring surface forces and films
- Cylindrical geometry is unrealistic
20
Cylindrical or Angular Pore Cross-section?
Sandstone
Clay
- Soil pore spaces are formed by aggregation of
primary particles and mineral surfaces, their
representation as angular pore cross-sections is
a more realistic model than cylindrical. - Angular pores allow dual-occupancy of wetting and
non-wetting phases.
21
Pore Shape and Saturation (Capillarity Only)
- Dual occupancy manifested by all angular pores
but not in cylindrical pores. - Various degrees of shape-dependent hysteresis
exhibited by angular pores. - Pores with higher angularity (e.g., triangle)
retain more liquid at a given potential than
pores with low angularity (e.g., hexagon).
22
Capillary Considerations in Angular Pores Made
Simple Mason and Morrow, 1991 Tuller et. al,
1999
where
Scanning electron micrographs of soils Blank and
Fosberg, 1989
23
Instantaneous snap-off
1.6
Glass-cell
Water
0.8
mm
Slit
0.0
24
Liquid Configurations in Square-Shaped Pores
During Drainage
1 mm
0.5 mm
25
Snap-Off Mechanisms in the Unit Cell
- Assuming continuity of all phases, we consider
pore and slit snap-off mechanisms (spontaneous
redistribution of liquid) within the unit cell. - Piston-like pore snap-off mechanisms are not
considered under the slow laminar flow regimes
26
Other Snap-Off Mechanisms in Porous Media
- Snap-off in pore throats with uncontrolled growth
of a perturbation due to capillary configuration
(Radke
27
Other Snap-Off Mechanisms in Porous Media
- Cavitation under tension (well documented in
plant xylem) could lead to spontaneous and rapid
emptying of pores (of a particular critical size)
28
SWC Calculation for a Unit Cell
- We distinguish two situations, before and after
slit snap off - Prior to slit snap off (mltmc)
- After slit snap off (mgtmc)
29
Angularity, Area, and Drainage Radius (Cn)
Factorsfor Different Regular polygon-Shaped Pores
30
Liquid-vapor interfacial areacalculation for a
unit cell
- Again, we distinguish between conditions before,
and after, slit snap off - Prior to slit snap off (mltmc)
- After slit snap off (mgtmc)
(where n4, Fn and An as defined for a square
pore)
31
Liquid-vapor interfacial areafor various unit
cells