Ethers, Epoxides and Sulfides - PowerPoint PPT Presentation
1 / 27
Title:
Ethers, Epoxides and Sulfides
Description:
Remember the alkoxy name is only a higher priority than the halides. ... American cockroach. Chapter 14. 6. 5. forming complexes. a. 6 electron reagents - storage ... – PowerPoint PPT presentation
Number of Views:122
Avg rating:3.0/5.0
Title: Ethers, Epoxides and Sulfides
1
Chapter 14 Ethers, Epoxides and Sulfides
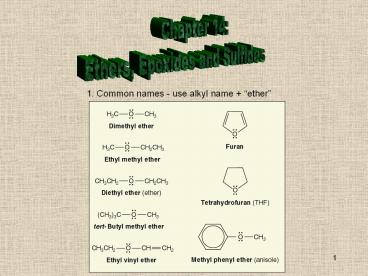
1. Common names - use alkyl name ether
2
2. IUPAC nomenclature - name the most complicated
part as the alkane and add the rest as an
alkoxy substituent (in alkyl name - drop yl
add oxy). Remember the alkoxy name is only a
higher priority than the halides. It is even
lower than the alkyl substiuent.
(actually the S,R enantiomer as drawn)
3. Cyclic ethers a. common names
3
b. IUPAC - two ways(!)
Name as an epoxide
Name as an oxirane
Best
4
B. Uses 1. solvents - have moderate polarity and
are unreactive (for the most part).
2. feedstock chemicals
3. polymers
5
4. biological - well see some more later
Juvenile hormone - prevents maturation of insect
larvae
Phermone for the American cockroach
6
5. forming complexes a. 6 electron reagents -
storage
b. crown ethers, etc.
7
(No Transcript)
8
C. Spectra 1. IR- nothing!! - BUT--if you know O
is present AND there are no CO or O-H
peaks 2. NMR
1H
d 3.5 - 4.0 ppm
d 65 - 90 ppm
13C
9
D. Synthesis of ethers 1. Industrial -
bimolecular dehydration a. overall reaction
(Rs must be the same)
b. mechanism
10
2. Williamson Synthesis a. overall reaction
NOTE RX really needs to be 1
11
3. Oxymercuration-demercuration a. overall
reaction
And so using ROH in place of H2O
b. mechanism issues
12
c. examples
E. Reactions of ethers - not many! 1. Cleavage
by strong acids a. overall
b. mechanism
(could be SN1 In some cases)
13
c. examples
2. Autoxidation a. overall reaction
b. partial mechanism
Stable free radical
14
NOW
heat given off!!!
BE VERY CAREFUL IN STORING ETHERS!!!
15
F. Sulfides 1. Nomenclature a. common - drop
ether add sulfide
b. IUPAC - replace alkoxy name with
alkylthio
2. Reactions a. oxidation
16
(No Transcript)
17
b. preparation - using thiols
c. reactions - substitution (SN2)
18
G. Epoxides 1. Preparation a. Reaction of
olefins with peracids
19
b. Reaction with halohydrins
20
2. Reactions of epoxides - ring strain
- Acid catalyzed ring opening
- general-
21
Note orientation
22
b. Ring opening under basic conditions
Note orientation
23
(No Transcript)
24
An aside into the land of bar-be-que!!
Bad reaction
arene oxides
OK reaction
25
(No Transcript)
26
H. Summary 1. Ethers a. nomenclature
spectroscopy b. preparation i. Williamson
synthesis ii. alkoxymercuration-demecuration
c. reactions i. acid catalysed cleavage ii.
auto-oxidation 2. Sulfides a.
nomenclature b. oxidation reactions to
sulfones, sulfoxides c. preparation - analog to
Williamson synthesis d. sulfides as
nucleophiles
27
3. Epoxides - synthesis a. reaction of olefins
with peracids b. cyclization of halohydrins 4.
Epoxides - reactions a. acid catalyzed ring
opening b. nucleophilic ring opening under basic
conditions