PFS
1 / 8
Title:
PFS
Description:
Inter-rater reliability and internal consistency not applicable to PPI ... Linear mixed effects model of within. patient variationa. Region effect 1% Country ... –
Number of Views:45
Avg rating:3.0/5.0
Title: PFS
1
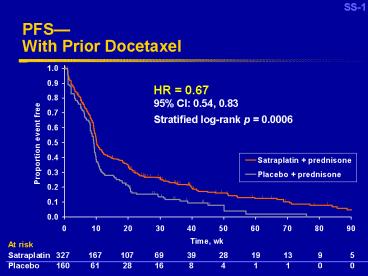
PFS With Prior Docetaxel
PFS curve data for prior docetaxel.xls T_4.1.1_E_P
FS_PrDoc.doc - DV
Figure 9
HR 0.6795 CI 0.54, 0.83 Stratified log-rank
p 0.0006
At risk
Satraplatin
Placebo
327 167 107 69 39 28 19 13 9 5
160 61 28 16 8 4 1 1 0 0
2
PFS Proportional Hazards Assumption
CumHazardPlot.xls Christian 6-29
CumHazardPlot.xls Christian 6-29 - DV
1.0
2.7
7.4
20.1
54.6
148.4
3
(No Transcript)
4
Topic 3 Draft FDA Guidance on Patient Reported
Outcome Measures - 2006
- If documentation exists that a single item is a
reliable and valid measure of the concept of
interest (eg, pain severity), a one-item PRO
instrument may be a reasonable measure to support
a claim concerning that concept.
5
Overall Survival by Baseline PPI
F_4.2.12.xls F_4.2.12_KM_OS_RespNoResp.doc
F_4.2L_KM_OS_ByPPI_at5wk.xls F_4.2L_KM_OS_ByPPI_a
t5.doc
p lt 0.0001
Treatment Total Dead Alive Median
PPI 0 340 124 216 77.0
PPI 1 268 140 128 55.1
PPI 2 220 129 91 45.0
PPI 3 98 56 42 50.3
PPI 4-5 17 12 5 29.9
6
Topic 3Psychometric Properties
- Single-item numeric pain measure
- Inter-rater reliability and internal consistency
not applicable to PPI - Test-retest (baseline - week 1) r 0.8, p lt
0.001 - Standard deviation of PPI in SPARC at baseline
(1.09) suggests 0.5 reduction/ increase on this
scale is a moderate effect (clinically
significant) - Prespecified 1-unit increase in PPI reflects
clinically significant pain progression
7
Topic 3 No Substantive Effect of Culture,
Language, or Region on PPI
- Linear mixed effects model of within patient
variationa - Region effect lt 1
- Country effect 1.2
- Site effect lt 5
a Accounting for baseline PS, baseline
analgesics, treatment effect, and between-patient
effects.
8
Prior Docetaxel (Duration, Time From)
Source
EF-119
Satraplatin prednisonen 615 Placebo prednisonen 313
Duration of prior docetaxel therapy 21 weeks 19 weeks
Time from docetaxel to randomization into SPARC 11 weeks 14 weeks