Solution Kinetics
1 / 4
Title: Solution Kinetics
1
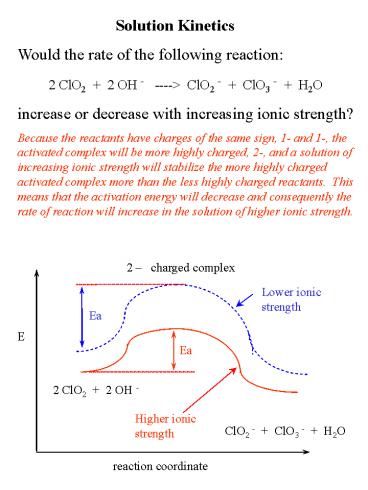
Solution Kinetics Would the rate of the
following reaction 2 ClO2 2 OH - ----gt
ClO2 - ClO3 - H2O increase or decrease
with increasing ionic strength? Because the
reactants have charges of the same sign, 1- and
1-, the activated complex will be more highly
charged, 2-, and a solution of increasing ionic
strength will stabilize the more highly charged
activated complex more than the less highly
charged reactants. This means that the
activation energy will decrease and consequently
the rate of reaction will increase in the
solution of higher ionic strength.
2
The reaction in this quiz can be represented 2 A
2 B- ------gt A2B22 - ------gt
products rate k A2B22 - (from
transition state theory) In this development the
solutions are treated as non-ideal and the
equilibrium constant expression for the
equilibrium between the reactants and activated
complex in terms of activities written as K
aA2B2 / ( a2A a2B- ) The activities are related
to concentrations through activity coefficients,
g ai g i i / co Here division by the
standard state concentration on the molar scale,
co 1 mole dm-3, insures that the activities are
unitless. Substitution into the equilibrium
constant expression gives K ( g
A2B2 A2B22 - / co ) / (g A A / co )2 ( g B
B- / co )2 (co)3 g A2B2 / (g 2A g 2B- )
A2B22 - / ( A2 B-2 ) Now substituting
for the concentration of the activated complex in
the rate equation yields rate ( k K /
(co)3 ) ( g 2A g 2B- / gA2B2 ) A2 B-2
which allows the rate constant to be identified
as k ( k K / (co)3 ) ( g 2A g 2B- / gA2B2
) ko ( g 2A g 2B- / gA2B2 ) where ko is
the rate constant in an infinitely dilute ideal
solution where the g i 1.
3
Assuming a dependence of activity coefficients on
ionic strength of the form log g i - C Zi2
f (I) gives log k log ko 2 log gA
2 log gB- - log gA2B2 log ko - C f
(I) 2 Z2A 2 Z2B- - ZA2B22 - , log
ko - C f (I) 2 (0)2 2 (-1) 2 - (-2) 2
log ko 2 C f (I) This equation
implies that as the ionic strength increases the
rate of the reaction will increase, just as our
shorter qualitative argument predicted.
4
Solution Kinetics Would the rate of the
following reaction 2 ClO2 2 OH - ----gt
ClO2 - ClO3 - H2O increase or decrease
with increasing ionic strength? Explain your
reasoning.