6' Ideal Liquid Solutions - PowerPoint PPT Presentation
1 / 8
Title:
6' Ideal Liquid Solutions
Description:
We have already developed a model for the chemical potential of ideal solutions. ... in earlier is known (in a slightly different form) as the Lewis-Randall equation: ... – PowerPoint PPT presentation
Number of Views:30
Avg rating:3.0/5.0
Title: 6' Ideal Liquid Solutions
1
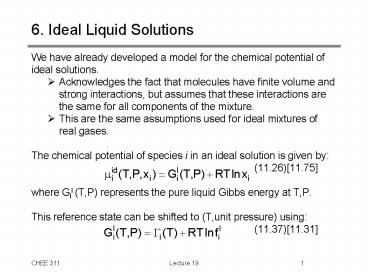
6. Ideal Liquid Solutions
- We have already developed a model for the
chemical potential of ideal solutions. - Acknowledges the fact that molecules have finite
volume and strong interactions, but assumes that
these interactions are the same for all
components of the mixture. - This are the same assumptions used for ideal
mixtures of real gases. - The chemical potential of species i in an ideal
solution is given by - (11.26)11.75
- where Gil (T,P) represents the pure liquid Gibbs
energy at T,P. - This reference state can be shifted to (T,unit
pressure) using - (11.37)11.31
2
Ideal Liquid Solutions
- Substituting for Gil (T,P) yields
- To estimate the chemical potential of component i
in an ideal liquid solution, all we require is
the composition (xi) and the pure liquid fugacity
(fil ). - The fugacity of a pure liquid can be calculated
using - (11.41)11.44
- For those cases in which the ideal solution model
applies, we require only pure component data to
estimate the chemical potentials and total Gibbs
energy of the liquid phase.
3
Non-Ideal Liquid Solutions
- Relatively few liquid systems meet the criteria
required by ideal solution theory. In most cases
of practical interest, molecular interactions are
not uniform between components, resulting in
mixture behaviour that deviates significantly
from the ideal case. - The approach for handling non-ideal liquid
solutions is exactly the same as that adopted for
non-ideal gas mixtures. We define a solution
fugacity, fil as - (11.42)11.46
- To use this approach, we require experimental
data or correlations pertaining to the specific
mixture of interest
4
Lewis-Randall Rule
- The ideal solution model developed in earlier is
known (in a slightly different form) as the
Lewis-Randall equation - (11.80)11.83
- The solution fugacity of component i in an ideal
solution (gas or liquid) can be represented by
the product of the pure component fugacity and
the mole fraction. - Whenever you apply an ideal solution model, you
are using the Lewis-Randall rule. - This is an approximation that yields reasonable
results for similar compounds (benzene/toluene,
ethanol/propanol) - However, it is important that you appreciate the
limitations of this rule. When you cannot find
mixture data, you may need to use it (but I
suggest you look harder).
5
Liquid Phase Activity Coefficients
- Based on our definition of solution fugacity
- (11.42)11.46
- we could define a liquid phase solution fugacity
coefficient - that reflects deviations of the solution fugacity
from a perfect gas mixture. - A more logical approach is to measure the
deviations of the solution fugacity from ideal
solution behaviour. For this purpose, we define
the activity coefficient - (11.87)11.90
- this convenient parameter is used to correlate
non-ideal liquid solution data, just as ?i is
used for gas mixtures
6
Excess Properties of Non-Ideal Liquid Solutions
- Most of the information needed to describe
non-ideal liquid solutions is published in the
form of the excess Gibbs energy, GE. - Excess properties are defined as the difference
between the actual property value of a solution
and the ideal solution value at the same T, P,
and composition. - ME(T,P, xn) M(T,P, xn) - Mid(T,P,
xn) (11.82)11.85 - In defining excess properties, we use ideal
solution behaviour as our reference. Pure
components cannot have excess properties. - Partial excess properties can also be defined
- MiE(T,P, xn) Mi(T,P, xn) - Miid(T,P,
xn)(11.86)11.88 - where
7
Excess Properties of Non-Ideal Liquid Solutions
- The partial excess Gibbs energy is of primary
interest - where the actual partial molar Gibbs energy is
provided by equation 11.42 11.46 - and the ideal solution chemical potential is
- Leaving us with the partial excess Gibbs energy
- (11.88)11.91
8
Excess Properties of Non-Ideal Liquid Solutions
- Why do we define excess properties for liquid
solutions? - They are more easily applied to experimental data
- Activity coefficients can be treated as partial
molar properties with respect to excess
properties. Three important results follow - (11.92)11.96
- The Gibbs-Duhem equation
- (11.14)
- The summability relation, providing GE from ln?i
data - (11.95)11.99






























![The Benefits of Tube Liquid Cold Plate [Infographic] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/10265504.th0.jpg?_=20250320062)