Ring current effects
1 / 14
Title: Ring current effects
1
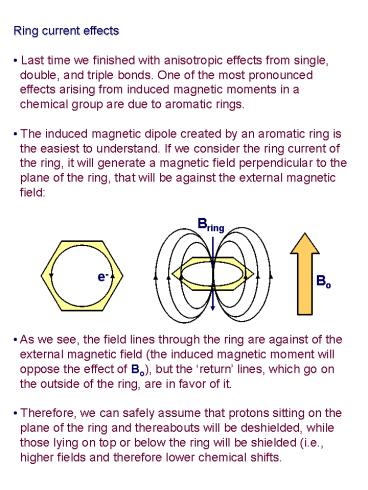
- Ring current effects
- Last time we finished with anisotropic effects
from single, - double, and triple bonds. One of the most
pronounced - effects arising from induced magnetic moments
in a - chemical group are due to aromatic rings.
- The induced magnetic dipole created by an
aromatic ring is - the easiest to understand. If we consider the
ring current of - the ring, it will generate a magnetic field
perpendicular to the - plane of the ring, that will be against the
external magnetic - field
Bring
e-
Bo
2
- Ring current effects (continued)
- As we had for simpler systems (single, double,
and triple - bonds), we can also estimate the degree of
shielding as a - function of the position of our nuclei around
the ring. - There are several formulas with different
degrees of - precision, but even the simplest ones give us a
pretty decent - estimate. The simplest one is the Polple
point-dipole model
H
r
q
drc Cpople irc r-3 . ( 1 - 3 . cos2q )
3
- Ring current effects ()
- As was the case for single, double, and triple
bonds, we can - plot the value of the shielding as a function
of the position in - space of the 1H under study. It will also be
cone-shaped, - with a shielding regions (-, lower chemical
shift), and - deshielding regions (, higher chemical shift)
- Protons on the sides of the aromatic ring will
feel a higher - local magnetic field (higher ppms), while
those on top or
7.27
7.79
7.41
4
- Ring current effects ()
- There are cases in which the protons of the ring
end up - inside the shielding cone of the aromatic ring,
such as in - 18annulene
- There is one last example of a ring with a
considerable - anisotropic effect. Cyclopropane is very
strained, and has - double bond character (carbons have sp2
character). There - is a magnetic dipole perpendicular to the plane
of the ring
9.28
-2.99
-
-
5
- Electric field and Van der Waals effects
- Although there are many other factors affecting
1H chemical - shifts, well finish by describing the effect
that polar groups - and close contacts have on shifts.
- We can understand pretty intuitively how a
charged group will - affect the shielding of a proton. Depending on
the charge, the - electric field will pull or push on the
electron density around - the proton, deforming it, and therefore
affecting the local field. - Analogously, an uncharged group that sits close
to the proton - will disturb its electron density due to van
der Waals - contacts. Both effects are appropriately
represented by the - Buckingham equation
C
Ds - AEC-H - BE2
H
6
- Some examples
- To conclude this discussion of factors affecting
chemical - shift, lets take a look at some interesting
examples in which - chemical shift can be used to decide on the
structure of - different molecules.
- The first one deals with cyclopropane
anisotropy. In the - following compound, the chemical shift of the
indicated - protons appears were expected for aromatic
protons - However, if we just change the two methyls for a
spiro
7.42
6.91
7
- Some examples (continued)
- In the following ketones, we can see the effects
of the - carbonyl group anisotropy
- Finally, the following example
- demonstrates that antiaromatic
- systems are paramagnetic (their
- induced field is in favor of the
- external magnetic field). In this
- dihydropyrene, the methyls show
- up were expected for an aromatic
7.27
6.57
5.47
d (CH3) -4 d (Ar-H) 8
d (CH3) 21 d (Ar-H) -4
8
- Some examples ()
- Another case in which several effects come into
play is seen - in a,b-unsaturated ketones. Here we resonance
(electronic - effects) dominating the shift at the b protons
- We also have CO group anisotropy
- In cis-malonates the deshielding is not as
strong because
6.28
6.83
-1.0
-2.4
9
- Shoolery chemical shift rules for 1H
- As we have seen, most of the different effects
on 1H - chemical shifts have been tabulated in one way
or another. - Furthermore, we also saw that most of the
effects are - additive, meaning that if we can estimate the
different effects - on the chemical shift of a certain 1H from
different groups - and bonds, we can in principle estimate its
chemical shift by - adding all the effects together.
- There are several empirical rules, derived
mostly by - Shoolery in the late 50s/early 60s.
- In order to use them, we first have to identify
the type of - proton we have, such as aliphatic CH3, CH2, CH,
olefinic - CH2 or CH, aromatic, a or b to a ketone or
alcohol, - belonging to an a a,b-unsaturated system, etc.
They will have - a base value.
dH dHbase S contributions
10
- Shoolery rules (continued)
- Aliphatic compounds. There are two approaches to
the - calculation of additive effects on the 1H
chemical shifts. - The first one is very simple. We just use two
skeletons with - two base values, R1-CH2-R2 or R1-CH-(R2)-R3,
and add the - effects from the R1, R2, or R3 groups
Substituent
d
Alkyl
0.0
-CC-
0.8
R1-CH2-R2 d 1.25 R1 R2
-C?C-
0.9
-C6H5
1.3
-CO-R
1.3
-OH
1.7
-O-R
1.5
R1-CH2-(R2)-R3 d 1.50 R1 R2 R2
-O-CO-R
2.7
-NH2
1.0
-Br
1.9
-Cl
2.0
11
- Shoolery rules ()
- The second method is pretty more general. We
start with - methane (d of 0.23 ppm), and then we add
substituent - effects directly.
- Now, if instead of a susbtituent we have another
carbon - chain, we have to consider how many carbons it
has, and - each carbon will have an increment we need to
add
CH3-
0.47
C6H5-
1.85
d 0.23 S S(d)
RO-
2.36
RC(O)O-
3.13
C3
0.248
0.244
0.147
0.006
C3
C2
C3
C2
C2
C3
C2
C3
C3
C1
C2
C3
HO-
2.47
0.048
0.235
Br-
1.995
0.363
0.023
CH3O-
-
-0.374
-
-O-CO-CR3
2.931
0.041
-0.086
12
- Shoolery rules ()
- Olefines. For alkenes we change the tables for
the base - values, but we also have to consider the
stereochemistry of - the substituent (cis, trans, or gem)
d 5.25 Rgem Rtrans Rcis
Substituent
dgem
dcis
dtrans
H-
0.0
0.0
0.0
Alkyl-
0.45
-0.22
-0.28
-OR
1.21
-0.60
-1.00
-COOH
0.80
0.98
0.32
-Ar
1.38
0.36
-0.07
-CC-
1.24
0.02
-0.05
-OH
1.22
-1.07
-1.21
-Cl
1.08
-0.40
-1.02
13
- Shoolery rules ()
- Aromatics. Finally, the Schoolery rules allow us
to calculate - the approximate chemical shifts in aromatic
compounds. - Again, we have a different base value of 7.26
(benzene).
d 7.26 Rortho Rmeta Rpara
Substituent
dortho
dmeta
dpara
H-
0.0
0.0
0.0
CH3-
-0.18
-0.10
-0.20
-NO2
0.95
0.26
0.38
-COOH
0.85
0.18
0.25
-OCH3
1.38
0.36
-0.07
-Cl
1.24
0.02
-0.05
-F
1.22
-1.07
-1.21
-CONH2
1.38
0.36
-0.07
-CHCH2
1.24
0.02
-0.05
-SO3H
1.22
-1.07
-1.21
14
- Shoolery rules ()
- For p-Xylene
- dHa 7.26 - 0.18 - 0.10 6.98 (6.97)
- dHb dHa
- For 1-Chloro-4-nitrobenzene
- dHa 7.26 0.95 - 0.02 8.19 (8.17)
- dHb 7.26 0.03 0.26 7.55 (7.52)
- For mesitylene