Proteins - PowerPoint PPT Presentation
1 / 45
Title:
Proteins
Description:
Classification, structure, and nomenclature of amino acids. Amino acid modifications ... Hydroxyproline (HYP) Disulfide bonds stabilize protein structures ... – PowerPoint PPT presentation
Number of Views:59
Avg rating:3.0/5.0
Title: Proteins
1
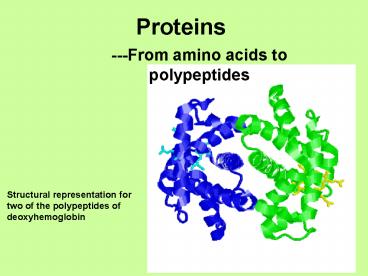
Proteins
- ---From amino acids to polypeptides
Structural representation for two of the
polypeptides of deoxyhemoglobin
2
Overview of lecture
- What are proteins
- Distance and time scales
- Basic amino acid structure
- L- vs. D-stereoisomers
- Classification, structure, and nomenclature of
amino acids - Amino acid modifications
- Peptide bond formation and structure
- Disulfide bond formation
3
(No Transcript)
4
(No Transcript)
5
What are proteins
- Proteins are linear polymers (chains) of amino
acids
Human insulin chain A
6
Amino acid
7
Asymmetric Carbon
- The asymmetric carbon of the AA
- All amino acid (except glycine) have chiral
centers. - Two kinds of AA, the L form and D form.
- All proteins in our body contain AA in L form.
Chiral carbon - A carbon atom with four different
substituents attached.
8
Ionization of AA
- COOH
- NH2
- R group
- Isoelectric point (pI)
9
Standard amino acid
- Twenty coding amino acid-DNA sequence
- New amino acids (2)
- No protein amino acids
- MW 110
10
There are 20 L-amino acids in protein, each with
unique side chains
11
Essential and Nonessential
- Of these 20 amino acids, 9 cannot be synthesized
by our body. - These 9 amino acids are therefore classified as
essential AAs and must be included as part of our
diet.. - Essential and nonessential Amino acids in Humans
- Essential
- Methionine Histidine Phenylalanine Isoleucin
e - Threonine Leucine Lysine
Valine - Arginine Tryptophan
12
L -Glyceraldehyde
L - Alanine
Amino Acids
13
(No Transcript)
14
GROUPS OF AMINO ACIDS
1. Aliphatic amino acids 2. Hydroxy amino
acids 3. Acidic amino acids 4. Amide amino
acids 5. Basic amino acids 6. Sulfur-containing
amino acids 7. Aromatic amino acids 8. Secondary
amino acids
15
Aliphatic Amino Acids
Glycine (GLY)
Alanine (ALA)
16
Aliphatic Amino Acids
Leucine (LEU)
Valine (VAL)
H
H
-
-
NH
C COO
NH
C COO
3
3
CH
2
CH
CH
CH
CH
3
CH
CH
3
3
3
17
Aliphatic Amino Acids
Isoleucine (ILE)
H
-
NH
C COO
3
CH
CH
CH
CH
2
3
3
18
Amino Acids with Alcohol
Serine (SER)
Threonine (THR)
H
H
-
NH
C COO
-
3
NH
C COO
3
CH
OH
OH
CH
2
CH
3
19
Acidic Amino Acids
Aspartic Acid (ASP)
Glutamic Acid (GLU)
H
H
-
NH
C COO
-
3
NH
C COO
3
CH
2
CH
2
CH
2
-
COO
-
COO
20
Amino Acids with Amides
Asparagine (ASN)
Glutamine (GLN)
H
H
-
NH
C COO
-
3
NH
C COO
3
CH
2
CH
2
CH
2
C NH
2
C NH
2
O
O
21
Basic Amino Acids
Lysine (LYS)
Arginine (ARG)
H
H
-
NH
C COO
3
-
NH
C COO
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
CH
2
NH
2
2
NH
3
NH C NH
2
22
Basic Amino Acids
Histidine (HIS)
H
-
NH
C COO
3
CH
2
NH
HN
23
Sulfuric Amino Acids
Cysteine (CYS)
Cystine (CYS-CYS)
H
-
NH
C COO
-
-
COO
3
COO
NH
CH CH
S S CH
CH NH
CH
3
2
2
3
2
SH
24
Sulfuric Amino Acids
Methionine (MET)
H
-
NH
C COO
3
CH
2
CH
2
S
CH
3
25
Aromatic Amino Acids
Phenylalanine (PHE)
Tyrosine (TYR)
26
Aromatic Amino Acids
Tryptophan (TRY)
27
Secondary Amino Acids
Proline (PRO)
Hydroxyproline (HYP)
28
Disulfide bonds stabilize protein structures
Ribonuclease catalyzes the hydrolysis of RNA
molecules.
29
Disulfide bond formation is reversible
30
The histidine side chain can serve as both a
proton donor and a proton acceptor
31
Some amino acids are modified after the protein
is synthesized
- Modifications that occur after the protein is
synthesized are called post-translational
modifications
32
Protein phosphorylation can increase or decrease
enzyme activity
33
Linkage of a glycosaminoglycan to protein
34
Amino acids are joined by peptide bonds
35
Proteins are polypeptide chains
- The beginning of the protein is known as the
amino-terminus and the end of the protein is
known as the carboxyl-terminus.
36
Peptide Bond
- Two amino acids linking - Dipeptide
- 3 amino acids - Tripeptide
- 5 - 30 amino acids - Polypeptide
- 30 - 50 - Proteins
Ala-Val
Ala-Val-Gly
Ala-Val-Gly-Tyr-Trp-Met-Glu-Lys
37
The peptide bond has partial double bond character
38
Peptide bonds can be either cis or trans
39
Interconversion of cis and trans prolines
requires breaking and reforming a covalent bond
- cis-trans isomerization of Pro is slow in the
absence of an enzyme (prolyl isomerase).
40
Angles of the chain
- Due to the partial double bond character of the
peptide bond, free rotation only occurs around
the f and y angles
41
Angles of the chain
42
The conformational angles
- Peptides are rigid
- There is rotational freedom at each Ca bond
- The N-Ca angle is called phi (f)
- The Ca-C angle is called psi (y)
- The AA sequence and the phi-psi angles completely
specify a three dimensional structure
43
Constraints on conformation
- Most phi-psi combinations cant occur because
they cause the side chain and main chain to
collide - Permitted combinations are called Ramachandran
plots
44
Glycines structural role
- Glycine (H side chain) has lots of freedom
- Good for creating unusual shapes
- Glycine is highly conserved among homologous
sequences
45
Summary
- Proteins are made up of chains of AAs
- L- vs. D-stereoisomers
- Classification of AAs into hydrophobic, acidic,
basic, polar, and aromatic - Know the amino acid side chains!
- Amino acids are joined by peptide bonds
- Peptide bond has partial double bond character,
giving the peptide bond a rigid character