IV' Optimize Lead Compound - PowerPoint PPT Presentation
1 / 29
Title:
IV' Optimize Lead Compound
Description:
Variation of Ring Size and Structure. 4. Change in substitution pattern ... 2.23 meperidine (Demerol) 2.24 dextropropoxyphene (Darvon) 2.25 methadone ... – PowerPoint PPT presentation
Number of Views:134
Avg rating:3.0/5.0
Title: IV' Optimize Lead Compound
1
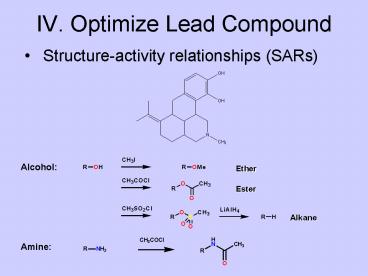
IV. Optimize Lead Compound
- Structure-activity relationships (SARs)
Alcohol
Amine
2
Alkene
Aromatic
No fit
3
IV. Optimize Lead Compound
Ketone
Ester
4
Esters as prodrugs
5
Amide
6
Carboxylic acids
7
Isosteres and Bioisosteres
Propranolol
8
Other functional groups
- Acid chlorides
- Anhydrides
- Alkyl halides
- Aryl halides
- Nitro groups
- Alkynes
- Thiols
- Nitriles
9
Example
Slightly reduced activity
Activity eliminated
Activity eliminated
10
IV. Optimize Lead Compound
- Analogs of pharmacophore
- Goals?
- 1. Variation of alkyl substituents
- 2. Variation of chain length
11
3. Extension of structure
12
Example
Adrenaline
Salbutamol (Ventolin) (Anti-asthmatic)
Propranolol (b-Blocker)
13
b-Adrenoceptor
14
b-Adrenoceptor
15
b-Adrenoceptor
SALBUTAMOL
16
a-Adrenoceptor
17
a-Adrenoceptor
18
a-Adrenoceptor
19
Variation of Ring Size and Structure
4. Change in substitution pattern
20
Variation of Ring Size and Structure
5. Variation in ring size
6. Variation in ring structure
21
7. Simplification
22
Procaine from Cocaine
23
8. Rigidification
24
Example
Less active
More active
25
Problems to Consider
- Pharmacokinetics
- Metabolism
- Prodrugs
- Synthesis/Manufacturing process
26
V. Toxicity Testing and Clinical Trials
- Testing
- Trials
- Phase 1
- Phase 2
- Phase 3
- Phase 4
27
VI. Market
28
Oxamniquine
- Case study
29
Pharmacophore
2.19 morphine (R R H) codeine (R CH3, R
H) heroin (R R COCH3)
2.20 levorphanol (R OH) 2.23 meperidine
(Demerol) 2.24 dextropropoxyphene
(Darvon) 2.25 methadone