Salinity - PowerPoint PPT Presentation
1 / 13
Title:
Salinity
Description:
Salinity may vary with seasons (dry/rain) Salinity variations. Location/type. Salinity ... Sea ice forming. Evaporation. Ocean buffering. Ocean pH = 8.1 ... – PowerPoint PPT presentation
Number of Views:146
Avg rating:3.0/5.0
Title: Salinity
1
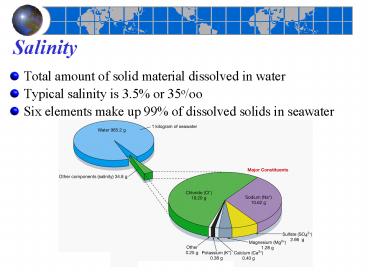
Salinity
- Total amount of solid material dissolved in water
- Typical salinity is 3.5 or 35o/oo
- Six elements make up 99 of dissolved solids in
seawater
Fig. 5.12
2
Constituents of ocean salinity
- Average seawater salinity 35
- Main constituents of ocean salinity
- Chloride (Cl)
- Sodium (Na)
- Sulfate (SO42)
- Magnesium (Mg2)
Figure 5-13
3
Measuring salinity
- Evaporation
- Chemical analysis
- Principle of constant proportions
- Major dissolved constituents in same proportion
regardless of total salinity - Measure amount of chlorine (chlorinity)
- Electrical conductivity
- Salinometer
4
(No Transcript)
5
Salinity
- Salinity total amount of solid material
dissolved in water - Can be determined by measuring water conductivity
- Typically expressed in parts per thousand ()
Figure 5-15
6
Pure water vs. seawater
7
Salinity variations
- Open ocean salinity 33 to 38 o/oo
- Coastal areas salinity varies more widely
- Influx of freshwater lowers salinity or creates
brackish conditions - Greater rate of evaporation raises salinity or
creates hypersaline conditions - Salinity may vary with seasons (dry/rain)
8
Salinity variations
9
How to change salinity
- Add water
- Remove water
- Add dissolved substances
- Remove dissolved substances
10
Surface salinity variation
- Pattern of surface salinity
- Lowest in high latitudes
- Highest in the tropics
- Dips at the Equator
- Surface processes help explain pattern
Figure 5-20
11
Processes affecting seawater salinity
- Processes that decrease seawater salinity
- Precipitation
- Runoff
- Icebergs melting
- Sea ice melting
- Processes that increase seawater salinity
- Sea ice forming
- Evaporation
12
Processes that add/subtract water
13
The hydrologic cycle
Figure 5-19
14
Surface salinity variation
- Pattern of surface salinity
- Lowest in high latitudes
- Highest in the tropics
- Dips at the Equator
- Surface processes help explain pattern
Figure 5-20
15
Processes affecting seawater salinity
- Processes that decrease seawater salinity
- Precipitation
- Runoff
- Icebergs melting
- Sea ice melting
- Processes that increase seawater salinity
- Sea ice forming
- Evaporation
16
Ocean buffering
- Ocean pH 8.1 (slightly basic)
- Buffering protects the ocean from experiencing
large pH changes
Figure 5-18