Structure%20of%20Matter - PowerPoint PPT Presentation
Title:
Structure%20of%20Matter
Description:
... atomic number and atomic weight for the 3 examples in the previous ? ... Types of Chemical Reactions. Electrolytes substances that release ions in water ... – PowerPoint PPT presentation
Number of Views:48
Avg rating:3.0/5.0
Title: Structure%20of%20Matter
1
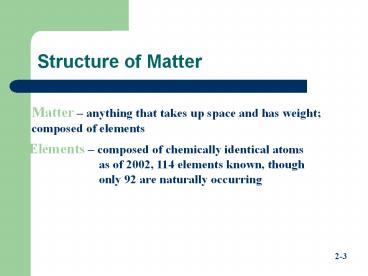
Structure of Matter
Matter anything that takes up space and has
weight composed of elements
Elements composed of chemically identical
atoms as of 2002, 114 elements known, though
only 92 are naturally occurring
2-3
2
CHNOPS
- Bulk elements include
- Carbon
- Hydrogen
- Nitrogen
- Oxygen
- Phosphorus
- Sulfur
3
Table 2.1a
4
Table 2.1b
5
Atoms
- Atoms smallest particle of an element
Atoms - composed of subatomic particles
protons carry a positive charge neutrons
carry no electrical charge electrons carry a
negative charge
6
Atomic Structure
- Nucleus
- central part of atom
- composed of protons and neutrons
- electrons move around the nucleus
2-4
7
Subatomic Particles
- Since the nucleus contains protons and neutrons,
the nucleus contains a positive charge - Generally, the number of electrons is equal to
the number of protons, so there is a net neutral
charge for the atom
8
Atomic Number and Mass Number
- Atomic Number
- number of protons in the nucleus of one atom
- each element has a unique atomic number
- equals the number of electrons in the atom
- Atomic Weight/Mass Number
- the number of protons plus the number of
neutrons in one atom - electrons do not contribute to the weight of the
atom
2-5
9
(No Transcript)
10
Question
- What is the atomic number and atomic weight for
the 3 examples in the previous slide?
11
Isotopes
- Isotopes
- atoms with the same atomic numbers but with
different atomic weights - atoms with the same number of protons and
electrons but a different number of neutrons - oxygen often forms isotopes (O16, O17, O18)
- unstable isotopes are radioactive they emit
subatomic particles
2-6
12
(No Transcript)
13
Question
- What is the atomic number and atomic weight for
the 3 isotope examples on the previous slide?
14
Molecules and Compounds
Molecules particle formed when two or more
atoms chemically combine
Compound particle formed when two or more atoms
of different elements chemically combine
Molecular formulas depict the elements present
and the number of each atom present in the
molecule H2 C6H12O6
H2O
2-7
15
Electrons
- found in regions of space called electron shells
(energy shells)
- each shell can hold a limited number of electrons
- for atoms with atomic numbers of 18 or less, the
following rules apply - the first shell can hold up to 2 electrons
- the second shell can hold up to 8 electrons
- the third shell can hold up to 8 electrons
- lower shells are filled first
- if the outermost shell is full, the atom is
stable inert
2-8
16
(No Transcript)
17
(No Transcript)
18
Octet Rule
- Atoms tend to interact so that they have 8
electrons to fill the outer shell
19
Ions
- Ion
- an atom that has gained or lost an electron(s)
- an electrically charged atom
- atoms form ions to become stable
- Cation
- a positively charged ion
- formed when an atom loses an electron(s)
- Anion
- a negatively charged ion
- formed when an atom gains an electron(s)
2-9
20
Ionic Bond
Ionic Bond
- an attraction between a cation and an anion
- formed when electrons are transferred from one
atom to another atom
2-10
21
(No Transcript)
22
Covalent Bond
Formed when atoms share electrons
- Hydrogen atoms form single bonds
- Oxygen atoms form two bonds
- Nitrogen atoms form three bonds
- Carbon atoms form four bonds
H ? H O O N N O C O
2-11
23
(No Transcript)
24
(No Transcript)
25
(No Transcript)
26
Structural Formulas
Structural formulas show how atoms bond and are
arranged in various molecules
2-12
27
Polar Molecules
- Polar Molecule
- molecule with a slightly negative end and a
slightly positive end - results when electrons are not shared equally in
covalent bonds - water is an important polar molecule
2-13
28
Hydrogen Bonds
- Hydrogen Bond
- a weak attraction between the positive end of
one polar molecule and the negative end of
another polar molecule - formed between water molecules
- important for protein and nucleic acid structure
2-14
29
Solution a mixture of one or more
substances called solutes, dispersed in a
dissolving medium called a solvent
Solutes Na Cl- Solvent H2O
30
- Most biological activities occur in aqueous
(water-based) solutions. - hydrophilic molecules dissolve in water
- hydrophobic molecules repel water
- amphipathic molecules -have both hydrophilic and
hydrophobic properties
31
Chemical Reactions
Chemical reactions occur when chemical bonds form
or break among atoms, ions, or molecules
Reactants are substances being changed by the
chemical reaction
Products are substances formed at the end of the
chemical reaction
NaCl Na Cl-
Reactant
Products
2-15
32
Types of Chemical Reactions
Synthesis Reaction chemical bonds are formed
A B AB
Decomposition Reaction chemical bonds are
broken AB A B
Exchange Reaction chemical bonds are broken and
formed AB CD AD CB
Reversible Reaction the products can change
back to the reactants A B n AB
2-16
33
(No Transcript)
34
(No Transcript)
35
Acids, Bases, and Salts
Electrolytes substances that release ions in
water
NaCl ? Na Cl-
Acids electrolytes that release hydrogen ions
in water
HCl ? H Cl-
Bases substances that release ions that can
combine with hydrogen ions
NaOH ? Na OH-
Salts electrolytes formed by the reaction
between an acid and a base
2-17
HCl NaOH ? H2O NaCl
36
Acid and Base Concentrations
pH scale - indicates the concentration of
hydrogen ions in solution
Neutral pH 7 indicates equal concentrations of
H and OH-
Acidic pH less than 7 indicates a greater
concentration of H
Basic or alkaline pH greater than 7 indicates
a greater concentration of OH-
2-18
37
(No Transcript)
38
Organic vs Inorganic
- Organic molecules
- contain C and H
- usually larger than inorganic molecules
- dissolve in water and organic liquids
- carbohydrates, proteins, lipids, and nucleic
acids
- Inorganic molecules
- generally do not contain C
- usually smaller than organic molecules
- usually dissolve in water or react with water to
release ions - water, oxygen, carbon dioxide, and inorganic
salts
2-19
39
Organic SubstancesCarbohydrates
- provide energy to cells
- supply materials to build cell structures
- water-soluble
- contain C, H, and O
- ratio of H to O close to 21 (C6H12O6)
- monosaccharides glucose, fructose
- disaccharides sucrose, lactose
- polysaccharides glycogen, cellulose
2-22
40
(No Transcript)
41
(No Transcript)
42
Organic SubstancesLipids
- soluble in organic solvents
- fats (triglycerides)
- used primarily for energy
- contain C, H, and O but less O than
carbohydrates (C57H110O6) - building blocks are 1 glycerol and 3 fatty acids
per molecule - saturated and unsaturated
2-24
43
Organic SubstancesLipids
- phospholipids
- building blocks are 1 glycerol, 2 fatty acids,
and 1 phosphate per molecule - hydrophilic and hydrophobic
- major component of cell membranes
2-25
44
(No Transcript)
45
Organic SubstancesLipids
- steroids
- connected rings of carbon
- component of cell membrane
- used to synthesize hormones
- cholesterol
2-26
46
Organic SubstancesProteins
- structural material
- energy source
- hormones
- receptors
- enzymes
- antibodies
- amino acids held together with peptide bonds
- building blocks are amino acids
2-27
47
(No Transcript)
48
(No Transcript)
49
Organic SubstancesProteins
Four Levels of Structure
2-28
50
(No Transcript)
51
Organic SubstancesNucleic Acids
- constitute genes
- play role in protein synthesis
- building blocks are nucleotides
- DNA (deoxyribonucleic acid) double
polynucleotide - RNA (ribonucleic acid) single polynucleotide
2-29
52
(No Transcript)
53
(No Transcript)
54
(No Transcript)
55
Table 2.6
56
Prokaryotes vs Eukaryotes