Introductory Chemistry
1 / 32
Title: Introductory Chemistry
1
Types of Matter
2
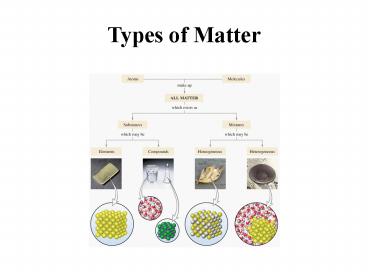
Pure Substances
- Elements-simplest pure substances, everything
else is made from these. Smallest unit is the
atom. - Compounds combinations of atoms in fixed
proportions. Cannot be separated by physical
means, but can by chemical means.
3
Mixtures
- Can separate by physical means.
- Homogeneous uniform composition, these are
solutions. - Heterogeneous uneven composition.
4
Physical Properties
- Physical properties
- -those that do not involve change in
composition. Subdivided - Extensive - depend on amount of matter, e.g.
mass, volume - Intensive independent of amount of matter,
e.g. melting point, density. - These can identify substances
5
Chemical Properties
- These that do involve a change in composition,
e.g. oxidation, decomposition, combustion.
6
Atoms Elements
- Elements-simplest pure substances, everything
else is made from these. - Cannot separate by physical means but can by
chemical means. Smallest unit is the atom. - Elements given symbols - either single upper case
letter or an upper case letter followed by a
lower case one. - E.g. C is carbon, O is oxygen, Co is cobalt.
7
Atoms Elements II
- Compounds-also pure substances, made from
combinations of atoms from two or more elements
in fixed proportions - Cannot separate by physical means, but can by
chemical means. Smallest units are molecules or
formula units. - Compounds given formulas that show how many of
each type of atom is present. - E.g. CO is carbon dioxide.
8
Daltons Atomic Theory
- 1. Matter composed of small, indivisible
particles called atoms. - 2. All atoms of one element are alike, but
differ from atoms of other elements. - 3. Compounds form when atoms of different
elements combine in fixed proportions. - 4. A chemical reaction involves a rearrangement
of atoms.
9
Cathode Rays
Cathode rays are the carriers of electric current
from cathode to anode inside a vacuumed
tube Cathode rays have the following
characteristics Emit from the cathode when
electricity is passed through an evacuated
tube Emit in a direction perpendicular to the
cathode surface Travel in straight lines Cause
glass and other materials to fluoresce Deflect in
a magnetic field similarly to negatively charged
particles
10
Canal Rays II
- If traces of a gas are left in a tube, positive
rays called canal rays are produced. - They can be deflected like cathode rays but in
the opposite direction the larger the atomic
mass of the gas the less they are deflected. - They are atoms that have lost an electron.
11
Nuclear Atoms
- Mass values for atomic particles and atoms are
based on the atomic mass unit (amu), defined as
1/12 the mass of an atom of carbon-12, see
isotopes below. - Particle Symbol Mass Charge Site
- Proton p 1 1 Nucleus
- Neutron no 1 0 Nucleus
- Electron e- .0005 -1 Outside nucleus
12
Nuclear Arithmetic
- Atomic number (Z) number of protons, all atoms
of an element have the same Z. Neutral atoms
have the same number of electrons as protons. - Mass number (A) sum of number of protons and
neutrons in an atom. - The number of neutrons is A Z
13
Isotopes
- Most elements have atoms with different numbers
of neutrons, therefore different A values These
called isotopes. AZX - Chlorine has two isotopes with A values 35 and
37 - 3517Cl (chlorine-35) and 3717Cl (chlorine-37)
14
Atomic Mass
- An average value for the mass of an atom in amu
based on relative amounts of each isotope. - Though strictly speaking incorrect, this is
commonly termed atomic weight. - Chlorine 75.0 of atoms are chlorine-35 and
25.0 chlorine-37 - The average atomic mass is obtained-
- Av.M. (35.0 x .750) (37.0 x .250) 35.5
15
Atomic Structure
- Atoms excited by heat or electricity emit light
of characteristic color (neon lights), several
colors. - - color due to frequency of light, blue is high -
high frequency means high energy. - - Bohr said electrons placed in "orbits" around
nucleus - - as they fall from one level to a lower one they
emit light - - only certain frequencies emitted, so only
certain orbits or energy levels exist.
16
The Bohr Model
17
Energy Levels
- Each energy level holds a definite maximum number
of electrons Where n is number assigned to
level e- 2n2 E.g. for level 3 e-
2(32) 18 - Each level is called a shell and has one or more
sub-shell and each sub-shell has one or more
orbitals, finally each orbital can hold a maximum
of two electrons.
18
Shells, Sub-shells Orbitals
- Shell Sub-shells Orbitals Electrons Shell Total
- 1 1s 1 2 2
- 2 2s 1 2
- 2p 3 6 8
- 3 3s 1 2
- 3p 3 6
- 3d 5 10 18
- 4 4s 1 2
- 4p 3 6
- 4d 5 10
- 4f 7 14 32
19
Electron Placement
- Electrons placed from lowest levels up according
to the value of Z with 1s being the lowest energy
level then 2s, 2p etc., but the energy level for
3d is higher than that for 4s which results in
this being filled first. Beyond this there are
more overlaps but we do not cover that here. The
orbitals have distinctive shapes and this in turn
leads to distinctive shapes for molecules.
20
Electron Configurations
- Element Shell 1 Shell 2 Shell 3 Shell 4 Orbital
config. - H 1e- 1s1
- He 2e- 1s2
- Li 2e- 1e- 1s22s1
- Be 2e- 2e- 1s22s2
- B 2e- 3e- 1s22s22p1
- C 2e- 4e- 1s22s22p2
- N 2e- 5e- 1s22s22p3
- O 2e- 6e- 1s22s22p4
- F 2e- 7e- 1s22s22p5
- Ne 2e- 8e- 1s22s22p6
- Na 2e- 8e- 1e- 1s22s22p63s1
- Mg 2e- 8e- 2e- 1s22s22p63s2
- Al 2e- 8e- 3e- 1s22s22p63s23p1
- Si 2e- 8e- 4e- 1s22s22p63s23p2
- P 2e- 8e- 5e- 1s22s22p63s23p3
- S 2e- 8e- 6e- 1s22s22p63s23p4
- Cl 2e- 8e- 7e- 1s22s22p63s23p5
- Ar 2e- 8e- 8e- 1s22s22p63s23p6
21
Electron Configurations III
- s-block Groups 1A 2A.
- Valence electrons in s subshell
- p-block Groups 3A - 8A.
- Valence electrons in p subshell
- d-block Groups 3B 2B.
- Valence electrons in s subshell
- f-block Lanthanides Actinides.
- Valence electrons in f subshell
22
Periodic Law
- Representative elements in the same group in the
periodic table owe their similarities in
properties to the fact that they all have the
same number of electrons in the outermost shell.
The group number is the same as the number of
electrons in the outermost shell.
23
General Locations
24
Periodic Table I
- When elements were arranged in order of
increasing atomic weight a pattern emerges in
which elements with similar properties occur at
definite intervals. Some irregularities occur,
but these are removed by placing the atoms in
order of atomic number this is now the basis for
the modern periodic table.
25
Periodic Table II
- 2. The columns are called groups. The ones with
B in the number are called transition elements,
they are all metals, and the Lanthanide and
Actinide series are called inner transition
elements. These will not be discussed in this
class.
26
Periodic Table III
- 3. The remaining groups are the representative
elements and are numbered 1A through 8A (also
sometimes 0). All the elements in one group have
the same number of electrons in the outer
(valence) shell which is the same as the group
number. They also have the same electron
configuration, this gives them many similar
properties.
27
Periodic Table IV
- 4.Some have names 1A - alkali metals, 2A -
alkaline earth metals, 7A - halogens and 8A -
inert (or noble) gases. From top to bottom in a
group elements become more metallic in nature - E.g group 4A C - nonmetal, Si - metalloid, Sn
and Pb - metals.
28
Periodic Table V
- 5.The rows are called periods and correspond to
the valence shell of the elements. They change
from left to right from metals to nonmetals. - 6.A zigzag line to the left of B, Si, Te At
separates metals from nonmetals. - 7.Metals form ionic bonds with nonmetals (next
chapter), they are shiny, conduct heat and
electricity and are strong.
29
Periodic Table VI
- 8.Nonmetals also form covalent bonds with each
other, physical properties are varied, they do
not conduct heat or electricity. - 9. Metalloids are Si, Ge, As, Sb, Te, Po At.
Their properties are intermediate between those
of metals and metalloids.
30
Periodic Properties I
- Atomic Radius Increases from top to bottom
within a group. This is due to the electrons in
outer shells being further from the nucleus.
Decreases from left to right along a period. This
is due to as more protons are added beyond a core
of a completed period, the electrons are held
more tightly by what is called the effective
nuclear charge. - Ions atoms can gain or lose electrons to get a
noble gas configuration.
31
Periodic Properties II
- Ionization energy energy required to remove an
electron. Decreases down a group as the furter
away from the nucleus the less tightly electrons
are held. Increases, with a few irregularities,
from left to right across a period because of the
increased effective nuclear charge
32
Periodic Properties III
- Electron Affinity energy change when an atom
gains an electron. Increases from left to right
across a period in parallel with the effective
nuclear charge. Top to bottom trends within a
group are not consistent.