Prezentacja programu PowerPoint
1 / 50
Title:
Prezentacja programu PowerPoint
Description:
2) diffusion through the bilayer core. 3) exit the membrane (potential barrier) ... The pore is lined by backbone amide groups and permits the transmembrane flux of ... –
Number of Views:24
Avg rating:3.0/5.0
Title: Prezentacja programu PowerPoint
1
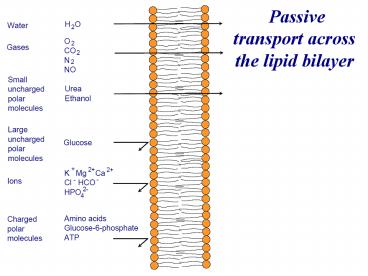
Passive transport across the lipid bilayer
2
Membrane permeability to nonelectrolytes
Steps (any can be rate limiting)
1) enter the membrane (potential barrier)
2) diffusion through the bilayer core
3) exit the membrane (potential barrier)
3
Diffusion of non-electrolytes
4
(No Transcript)
5
(No Transcript)
6
P in membranes is strongly correlated with Kp for
nonpolar solvent
7
Unstirred Layers
Molecule diffusion across the aqueous layers
adjacent to either surface of the membrane.
1 µm to 500 µm thickness.
- It is most prominent for relatively nonpolar
compounds the diffusion across the membrane
itself will be relatively fast.
- For water soluble compounds diffusion across
the unstirred layers will have relatively less
effect.
8
(No Transcript)
9
(No Transcript)
10
Osmosis
It is entropic in nature
11
The osmotic pressure difference can only arise if
there is a physical object, the semipermeable
membrane, present to apply force to the solute
particles.
12
Osmotically active solutes which cant diffuse
through the semipermeable membrane.
Easy way to measure osmolality Each Osm (of any
solute) lowers the freezing point of water by
2o C
13
Solutes Decrease the Chemical Potential of Water
There is a net water flow from compartment (2)
to compartment (1).
14
Osmotic Equilibrium
15
The osmolarity of a solution is equal to the
molarity of the particles dissolved in it.
1. 10 mmoles/liter of glucose 10 mosmoles/liter.
2. 10 mmoles/liter of NaCl 20 mosmoles/liter.
3. 10 mmoles/liter of CaCl2 ???
In a simple solutions the effect is additive.
16
Osmotic Flow
Water flows from the solution with a low osmotic
pressure to the solution with a high osmotic
pressure.
17
Osmotic pressure creates a depletion force
between large molecules
The depletion interaction molecular crowding
- Each of the large objects is surrounded by a
depletion zone of thickness equal to the radius a
of the small particles the centers of the small
particles cannot enter this zone.
- The depletion zone reduces the volume available
to the small particles eliminating it would
increase their entropy and hence lower their free
energy.
The depletion interaction is short range (lt2a)
18
(No Transcript)
19
Important summary points about osmosis
1. The steady-state volume of the cell is
determined by the concentrations of impermeant
ions.
2. Permeant solutes redistribute according to the
rules of electrodiffusion, and hence affect only
the transient volume of the cell.
20
Volume regulation of living animal cells
21
Osmoconformers animals, like sea slugs, that
allow the osmolarity of their internal
environment to change with that of the external
environment.
Osmoregulators animals that do not allow the
osmolarity of their internal environment to
change.
22
The activation energy (Ea) required for water
diffusion in an entirely aqueous environment 5
kcal/mol.
The activation energy (Ea) required for water
diffusion through the lipid bilayer 10-20
kcal/mol.
Diffusion through lipid bilayers slow, but
enough for many purposes
23
(No Transcript)
24
(No Transcript)
25
Ion transport across the cell membrane underlies
cellular Homeostasis and electrical activity.
Control of ion flux in response to external
stimulation, generates the fundamental signaling
step.
- the regulation of heart beat
- movement of muscle
- regulation of hormone release from pancreatic
cells
- the generation of thought
26
(No Transcript)
27
The ion transport across membrane depends on
Concentration profile
Electric potential
Free energy profile
28
The movement of ions.
29
How much K would flow out to establish a Vm of
-60 mV in an idealized cubic cell with edge
dimension of 10µm and a membrane permeable only
to K?
(C/cm2) surface area/(V Faraday constant)
3.7 10-18 moles
This is only 1 out of 30,000 K in the cell.
30
Capacitance of biological membranes 1 µF/cm2.
31
Comments
Nerst equation gives the value of membrane
potential ?nerst at which the ion is in
steady-state equilibrium.
At this value of ?nerst, the electrostatic energy
per mole (zF?m) is exactly counterbalanced by the
chemical energy per mole (RTln(ci/co)).
The value of Vm is independent of the
concentration or voltage profile within the
membrane!
32
Permeation of electrolytes
33
(No Transcript)
34
(No Transcript)
35
The concentration equalization can be
circumvented when
1) The transported substances may be bound by a
macromolecule inside the cell, e.g., O2 binding
by hemoglobin.
3) There are thermodynamically favorable process
which are coupled to transport active transport.
36
Equilibria of weak acids and weak bases
- At neutral pH, weak acids and weak bases are
predominantly in their charged forms (A- and BH).
- The charged species do not permeate across the
membranes hydrophobic barrier.
- The charged species are in equilibrium with
uncharged species that will permeate the membrane.
- The uncharged species (B) will reach the
equilibrium (Bo Bi).
37
(No Transcript)
38
(No Transcript)
39
- Like enzymes - bind and transport substrate
molecules, ONE at a time.
- Specific
- Dependent on temperature
- Can be inhibited
- Fast the flow may approach diffusion limit
e.g. 107 ions/sec.
40
Molecule must shed their water of hydration
before they can cross the membrane
Amino acid residues of the transporter interact
with "dehydrated" solute ?
Forming hydrophilic passageway or package through
membrane ?
41
They are hydrophobic compounds which can complex
an ion and carry it across a lipid bilayer.
42
Protonophores
43
With protonophores, electron transport proceeds
(NADH is oxidized, O2 is reduced) but no ATP is
synthesized. The mitochondria are uncoupled.
44
- Binds K in central cavity by CO coordination,
shields charge.
- Relatively slow rate of K transfer, 103 K/sec
per molecule
45
The valinomycin surronds the potassium ion with a
hydrophobic surface which allows the ion to cross
the membrane.
- It creates a membrane potential by transporting
capacitative charge.
- It depends on the membrane potential.
46
The selectivity of valinomycin for K
K binds tightly, but affinities for Na and Li
are about a 10 000 -fold lower.
Factor 1 Ionic radius (K gt Na gt Li) The
smaller Na ion cannot simultaneously interact
with all 6 oxygen atoms within valinomycin (Na
0.95 Å, K 1.32 Å).
47
- It is a K/H exchanger.
48
Alamethicin A Weakly Selective Channel
- Multi-conductance level channels,
- Rapid switching between conductance levels,
- Weakly cation selective (ca. 41 cationsanions)
49
- Unusual wide helix in membrane - 6.3 residues
per turn with a central hole - 4 Å diameter (a
?63 helix, NOT an ?-helix)
- The pore is ? 28 Å long and 4 Å in diameter when
a dimer forms.
- The pore is lined by backbone amide groups and
permits the transmembrane flux of small
monovalent cations (Na, K, H).
50
Gramicidin pore
- At high gramicidin overall transport rate
depends on gramicidin2.
51
(No Transcript)