Enhanced Photoefficiency of Immobilized TiO2 Catalyst
1 / 1
Title:
Enhanced Photoefficiency of Immobilized TiO2 Catalyst
Description:
Anodic polarization is capable of growing thick, crystalline, nanoporous and ... Anodic bias is also capable of reducing electron/hole pair recombination process ... –
Number of Views:153
Avg rating:3.0/5.0
Title: Enhanced Photoefficiency of Immobilized TiO2 Catalyst
1
Enhanced Photo-efficiency of Immobilized TiO2
Catalyst N. Baram1, D. Starosvetsky1, J.
Starosvetsky2, M. Epshtein2, R. Armon2, Y.
Ein-Eli1 Department of Materials Engineering1,
Environmental and Civil Engineering2,
Technion-Israel Institute of Technology, Haifa
32000, Israel
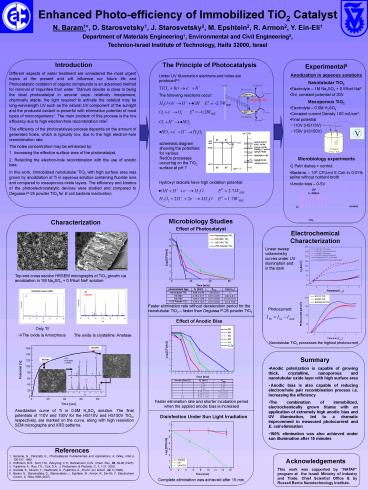
- Experimental5
- Anodization in aqueous solutions
- Nanotubular TiO2
- Electrolyte 1M Na2SO4 0.5wt NaF
- 2hr, constant potential of 20V.
- Mesoporous TiO2
- Electrolyte 0.5M H2SO4
- Constant current Density 100 mA/cm2.
- Final potential
- - 110V (HS110V)
- - 150V (HS150V)
- Microbiology experiments
- 2 Petri dishes control.
- Bacteria 106 CFU/ml E.Coli in 0.01 saline
without nutrient broth. - Anodic bias 0-5V
Microbiology Studies
Characterization
Effect of Photocatalyst
Electrochemical Characterization
Linear sweep voltammetry curves under UV
illumination and in the dark
Top and cross section HRSEM micrographs of TiO2
growth via anodization in 1M Na2SO4 0.5wt NaF
solution
Faster elimination rate without deceleration
period for the nanotubular TiO2 faster than
Degussa P-25 powder TiO2
Photocurrent
Effect of Anodic Bias
Only Ti! ?The oxide is Amorphous
The oxide is crystalline Anatase
Nanotubular TiO2 possesses the highest
photocurrent
- Summary
- Anodic polarization is capable of growing thick,
crystalline, nanoporous and nanotubular oxide
layer with high surface area - Anodic bias is also capable of reducing
electron/hole pair recombination process i.e.
increasing the efficiency - The combination of immobilized, electrochemically
grown titania with an application of extremely
high anodic bias and UV illumination, led to a
dramatic improvement in measured photocurrent and
E. coli elimination - 100 elimination was also achieved under sun
illumination after 15 minutes
Faster elimination rate and shorter incubation
period when the applied anodic bias is increased
Anodization curve of Ti in 0.5M H2SO4
solution. The final potentials of 110V and 150V
for the HS110V and HS150V TiO2, respectively, are
marked on the curve, along with high resolution
SEM micrographs and XRD patterns.
Disinfection Under Sun Light Irradiation
References
- Serpone, N., Pelizzetti, E., Photocatalysis
Fundamentals and Applications, A. Wiley, USA p.
126-157, 1989. - Hoffmann, M.R., Scot, T.M., Wonyong, C.H.,
Bahnemann, D.W., Chem. Rev., 95, 69-96 (1995). - Fujishima. A., Rao, T.N., Tryk, D.A., J.
Photochem. Photobio. C, 1, 1-21, 2000. - Sunada, K., Kikuchi, Y., Hashimoto, K.,
Fujishima, A., Enviro. Sci. Tech., 32, 5 (1998). - Baram, N., Starosvetsky, D., Starosvetsky, J.,
Epshtein, M., Armon, R., Ein-Eli, Y.,
Electrochem. Comm., 9, 1684-1688 (2007).
Complete elimination was achieved after 15 min.