Definition of Acid - PowerPoint PPT Presentation
1 / 26
Title:
Definition of Acid
Description:
The most common weak acid is acetic acid: HC2H3O2 (aq) H2O(l) H3O (aq) C2H3O2-(aq) ... soda (sodium bicarbonate) reacting with acetic acid in vinegar to give ... – PowerPoint PPT presentation
Number of Views:31
Avg rating:3.0/5.0
Title: Definition of Acid
1
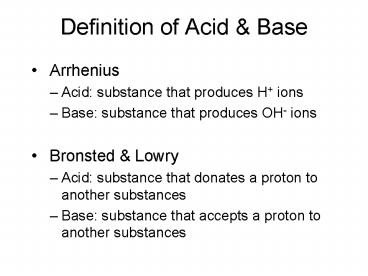
Definition of Acid Base
- Arrhenius
- Acid substance that produces H ions
- Base substance that produces OH- ions
- Bronsted Lowry
- Acid substance that donates a proton to another
substances - Base substance that accepts a proton to another
substances
2
Acids - A Group of Covalent Molecules Which Lose
Hydrogen Ions to Water Molecules in
Solution
When gaseous hydrogen iodide dissolves in water,
the attraction of the oxygen atom of the water
molecule for the hydrogen atom in HI is greater
that the attraction of the of the iodide ion for
the hydrogen atom, and it is lost to the water
molecule to form an hydronium ion and an iodide
ion in solution. We can write the hydrogen atom
in solution as either H(aq) or as H3O(aq) they
mean the same thing in solution. The presence of
a hydrogen atom that is easily lost in solution
is an Acid and is called an acidic solution.
The water (H2O) could also be written above the
arrow indicating that the solvent was water in
which the HI was dissolved.
HI(g) H2O(L) H(aq) I
-(aq)
HI(g) H2O(L) H3O(aq) I
-(aq)
H2O
HI(g) H(aq) I
-(aq)
3
(No Transcript)
4
Figure 4.8B Red cabbage juice added to solutions
in the beakers.Photo courtesy of James Scherer.
5
Reaction of nitric acid with water.
HNO3(aq) H2O(l) ? NO3-(aq) H3O(aq)
6
Molecular representation of ammonium hydroxide.
NH3(aq) H2O(l) ? NH4(aq) OH-(aq)
7
Strong Acid or Base An acid or base that ionizes
completely in water. It is present entirely as
ions it is a strong electrolyte.
8
- Weak Acid or Base
- An acid or base that is only partly ionized in
water. It is present primarily as molecules and
partly as ions it is a weak electrolyte. Weak
bases are often nitrogen bases such as NH3 - NH3(aq) H2O(l) ?NH4(aq) OH-(aq)
- The most common weak acid is acetic acid
- HC2H3O2 (aq) H2O(l) ? H3O(aq) C2H3O2-(aq)
- If an acid or base is not strong, it is weak.
9
(No Transcript)
10
- Polyprotic Acid
- An acid that results in two or more acidic
hydrogens per molecule - For example
- H2SO4, sulfuric acid
11
- Neutralization Reaction
- A reaction of an acid and a base that results in
an ionic compound (a salt) and possibly water
- Write the molecular, ionic, and net ionic
equations for the neutralization of sulfurous
acid, H2SO3, by potassium hydroxide, KOH.
Sulfurous acid is a WEAK acid.
12
- Molecular Equation
- (Balance the reaction and include state symbols)
- H2SO3(aq) 2KOH(aq) ? 2H2O(l) K2SO3(aq)
- Ionic Equation
- H2SO3(aq) 2K(aq) 2OH-(aq) ?
- 2H2O(l) 2K(aq) SO32-(aq)
- Net Ionic Equation
- H2SO3(aq) 2OH-(aq) ? 2H2O(l) SO32-(aq)
13
- Acid-Base Reaction with Gas Formation
- Some salts, when treated with an acid, produce a
gas. Typically sulfides, sulfites, and carbonates
behave in this way producing hydrogen sulfide,
sulfur trioxide, and carbon dioxide, respectively.
The photo shows baking soda (sodium bicarbonate)
reacting with acetic acid in vinegar to give
bubbles of carbon dioxide.
Write the reaction thats occurring.
14
(No Transcript)
15
Molarity (Concentration of Solutions) M
Moles of Solute Moles Liters of
Solution L
M
solute material dissolved into the solvent In
air , Nitrogen is the solvent and oxygen, carbon
dioxide, etc.
are the solutes. In sea water , Water is the
solvent, and salt, magnesium chloride, etc.
are the solutes. In
brass , Copper is the solvent (90), and Zinc is
the solute(10)
16
Fig. 3.11
17
Preparing a Solution - I
- Prepare a solution of Sodium Phosphate by
dissolving 3.95g of Sodium Phosphate into water
and diluting it to 300.0 ml or 0.300 l ! - What is the Molarity of the salt and each of the
ions? - Na3PO4 (s) H2O(solvent) 3 Na(aq)
PO4-3(aq)
18
- You place a 1.62-g of potassium dichromate,
K2Cr2O7, into a 50.0-mL volumetric flask. You
then add water to bring the solution up to the
mark on the neck of the flask. What is the
molarity of K2CrO7 in the solution?
Molar mass of K2Cr2O7 is 294 g.
0.110 M
19
- Dilution
- When a higher concentration solution is used to
make a less-concentration solution, the moles of
solute are determined by the amount of the
higher-concentration solution. The number of
moles of solute remains constant. - MiVi MfVf
- Note
- The units on Vi and Vf must match.
20
- A saturated stock solution of NaCl is 6.00 M. How
much of this stock solution is needed to prepare
1.00-L of physiological saline soluiton (0.154 M)?
21
- Titration
- A procedure for determining the amount of
substance A by adding a carefully measured volume
with a known concentration of B until the
reaction of A and B is just complete
22
Figure 4.22C Titration of an unknown amount of
HCl with NaOH (3). Photo courtesy of American
Color.
23
- Titration of an unknown amount of HCl with NaOH.
- In the titration above, the indicator changes
color to indicate when the reaction is just
complete.
24
- Zinc sulfide reacts with hydrochloric acid to
produce hydrogen sulfide gas - ZnS(s) 2HCl(aq) ?ZnCl2(aq) H2S(g)
- How many milliliters of 0.0512 M HCl are required
to react with 0.392 g ZnS?
25
- Molar mass of ZnS 97.47 g
- 0.157 L 157 mL HCl solution
26
(No Transcript)