Electrolysis of an aqueous solution and electrodes may react - PowerPoint PPT Presentation
1 / 5
Title:
Electrolysis of an aqueous solution and electrodes may react
Description:
Electrolysis of an aqueous solution and electrodes may react. eg: CuSO4 ... Bauxite. Aluminum Oxide. Heat. Al2O3 has a very high melting point: 2045 ... – PowerPoint PPT presentation
Number of Views:162
Avg rating:3.0/5.0
Title: Electrolysis of an aqueous solution and electrodes may react
1
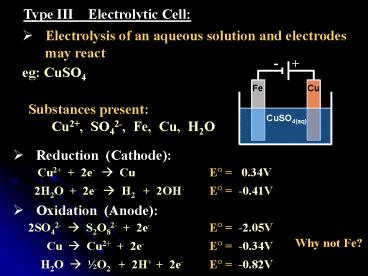
Type III Electrolytic Cell
- Electrolysis of an aqueous solution and
electrodes may react
eg CuSO4
Substances present Cu2, SO42-, Fe, Cu, H2O
- Reduction (Cathode)
Cu2 2e- ? Cu
E? 0.34V
2H2O 2e- ? H2 2OH-
E? -0.41V
- Oxidation (Anode)
2SO42- ? S2O82- 2e-
E? -2.05V
Why not Fe?
Cu ? Cu2 2e-
E? -0.34V
H2O ? ½O2 2H 2e-
E? -0.82V
2
Note
- Net voltage is 0V.
- Fe does not react because of its position.
- No net reaction occurs.
- This process is used for electroplating
3
- Industrial Applications of Electrolytic Cells
- Production of Cl2 (N. Vancouver)
Inert electrodes
Brine (solution of NaCl)
Anode
2Cl- ? Cl2(g) 2e-
Cathode
2H2O 2e- ? H2 2OH-
Products
Cl2
Bleach
H2
NH3 (ammonia)
NaOH
Soap
4
- Electrorefining
eg Production of pure copper.
Impure Copper (Copper Ore)
Pure Copper
Anode
Cu(s) ? Cu2 2e-
Impure
Cathode
Cu2 2e- ? Cu(s)
Pure
5
- Production of Aluminum
Heat
Al2O33H2O ? Al2O3 3H2O
Bauxite
Aluminum Oxide
- Al2O3 has a very high melting point 2045?C
- Instead of melting it, it is dissolved in
cryolite (Na3AlF6) at 1000?C
- The Al2O3 dissociates into Al3 and O2-
Carbon electrode
Al Core
Anode
C(s) 2O2- ? CO2(s) 4e-
Cathode
Al3 3e- ? Al(l)
Pure molten aluminum