The Chemical Basis of Life - PowerPoint PPT Presentation
1 / 54
Title:
The Chemical Basis of Life
Description:
Catabolism. Chemical bonds broken; energy released. ... Carbon dioxide (CO2): produced during the catabolism of organic compounds. ... – PowerPoint PPT presentation
Number of Views:91
Avg rating:3.0/5.0
Title: The Chemical Basis of Life
1
The Chemical Basis of Life
2
Basic Chemistry
- Matter, Mass, and Weight
- Matter anything that occupies space and has mass
- Mass the amount of matter in an object
- Weight the gravitational force acting on an
object of a given mass - Elements and Atoms
- Element the simplest type of matter with unique
chemical properties composed of atoms of only
one kind - Atom smallest particle of an element that has
chemical characteristics of that element
3
Atomic Structure
- Atoms composed of subatomic particles
- Neutrons no electrical charge
- Protons one positive charge
- Electrons one negative charge
- Nucleus formed by protons and neutrons
- Most of the volume of an atom occupied by
electrons
4
Atomic Number and Mass Number
- Atomic Number equal to number of protons in each
atom which is equal to the number of electrons - Mass Number number of protons plus number of
neutrons
5
Isotopes and Atomic Mass
- Isotopes two or more forms of same element with
same number of protons and electrons but
different neutron number - For example there are three types of hydrogen
- Denoted by using symbol of element preceded by
mass number as 1H, 2H, 3H - Atomic Mass average mass of naturally occurring
isotopes
6
Atomic Structure
- Central nucleus with electrons around it
- Several Energy Levels (2,8, ,)
- Octet rule
- Stable and reactive atoms
7
(No Transcript)
8
Periodic Table
- Atomic number
- Symbol
- Molecular weight
9
Chemical Reactions
- Two atoms share one or more electrons which helps
the reach the octet requirement and be stable
- The bonded atoms make up a molecule. Whether it
they are the same or different element
- The shared electrons can be shared equally
between the two atoms (covalent bond) or may be
taken predominantly by one of them leaving the
new molecule with some electric charge (Ionic
bond)
10
Chemical Bonds
- The chemical bonds need energy to be created. By
breaking a bond the energy of the bond is released
- The covalent bonds can be simple, double or
triple. The amount of energy of every bond is
additive
- Hydrogen bonds partial bond among charged ions.
Much weaker than then former bonds
11
Electrons and Chemical Bonding
- Intramolecular bonding occurs when outermost
electrons are either shared with or transferred
to another atom - Ionic Bonding atoms exchange electrons
- Covalent Bonding two or more atoms share
electron pairs - Ion an atom loses or gains electrons and
becomes charged - Cation positively charged ion
- Anion negatively charged ion
- In an ionic bond, cations and anions are
attracted to each other and remain close to each
other
12
(No Transcript)
13
Covalent Bonding
- Atoms share one or more pairs of electrons
- Single covalent two atoms share one pair of
electrons - Double covalent Two atoms share 4 electrons
- Nonpolar covalent Electrons shared equally
because nuclei attract the electrons equally - Polar covalent Electrons not shared equally
because one nucleus attracts the electrons more
than the other does
14
Molecules and Compounds
- Molecules and compounds two or more atoms
chemically combine to form an independent unit - Example a hydrogen molecule (H2), water (H2O)
- Molecular Mass determined by adding up atomic
masses of its atoms or ions - Example NaCl (22.99 35.45)
15
Intermolecular Forces
- Forces between molecules
- Result from weak electrostatic attractions
between oppositely charged parts or molecules, or
between ions and molecules - Weaker than forces producing chemical bonding
16
Intermolecular Forces Hydrogen Bonds
- Occur when the positively charged H of one
molecule is attracted to the negatively charged
O, N or F of another molecule - For example, in water the positively charged
hydrogen atoms of one water molecule bond with
the negatively charged oxygen atoms of other
water molecules - Hydrogen bonds play an important role in
determining the shape of complex molecules
17
(No Transcript)
18
Intermolecular Forces Solubility and
Dissociation
- Solubility ability of one substance to dissolve
in another - For example, sugar or salt dissolves in water
- Dissociation or Separation in ionic compounds,
cations are attracted to negative end and anions
attracted to positive end of water molecules the
ions separate and each becomes surrounded by
water molecules - Electrolyte dissociation of an ionic compound in
water
19
Electrolytes and Nonelectrolytes
- Electrolytes solutions made by the dissociation
of cations () and anions (-) in water - Have the capacity to conduct an electric current
- Currents can be detected by electrodes
- Nonelectrolytes solutions made by molecules that
dissolve in water, but do not dissociate do not
conduct electricity
20
Chemical Reactions
- Atoms, ions, molecules or compounds interact to
form or break chemical bonds - Reactants substances that enter into a chemical
reaction. - Products substances that result from the
reaction - Chemical bonds are made (synthesis anabolism)
and broken (decomposition catabolism) during
chemical reactions - Metabolism collective term used for the sum of
all of the anabolic and catabolic reactions in
the body
21
Synthetic Reactions
- Two or more reactants chemically combine to form
a new and larger product. Anabolism. - Chemical bonds made energy stored in the bonds.
- Responsible for growth, maintenance and repair
- Hydrolysis synthetic reaction where water is a
product - Produce chemicals characteristic of life
carbohydrates, proteins, lipids, and nucleic acids
22
Decomposition Reactions
- A large reactant is broken down to form smaller
products. Catabolism. - Chemical bonds broken energy released.
- Hydrolysis water is split into two parts that
contribute to the formation of the products - Example the breakdown of ATP to form ADP and
inorganic phosphate with a concomitant release of
free energy
23
Reversible Reactions
- Chemical reactions in which the reaction can
proceed either from reactants to products or from
products to reactants. - Equilibrium rate of product formation is equal
to rate of reactant formation - Example CO2 and H formation in plasma
24
Oxidation-Reduction Reactions
- Oxidation loss of an electron by an atom
- Reduction gain of an electron by an atom
- Oxidation-Reduction Reactions the complete or
partial loss of an electron by one atom is
accompanied by the gain of that electron by
another atom - Synthetic/decomposition reactions can be
oxidative reduction reactions - Reactions can be described in more than one way
25
Energy the capacity to do work
- Potential Energy energy stored in chemical
bonds energy that could do work if it were
released. Breaking chemical bonds releases
energy. - Kinetic Energy does work and moves matter
- Mechanical Energy energy resulting from the
position or movement of objects - Chemical Energy form of potential energy in the
chemical bonds of a substance - Heat Energy energy that flows between objects of
different temperatures
26
ATP and Potential Energy
27
Heat Energy
- When a chemical bond is broken and energy is
released, only some of that energy is used to
manufacture ATP. - Energy that is released but not captured is
released as heat. - The heat used by humans to maintain body
temperature.
28
Speed of Chemical Reactions
- Temperature affects rate of reaction.
- Increase in temperature means increase of kinetic
energy. - Molecules move faster, collide harder and more
frequently. - Concentration of reactants.
- As concentration of reactants increases, rate of
reaction increases. - A decrease of O2 in cells can cause death as rate
of aerobic chemical reactions decreases. - Catalysts substances that increase the rate of
chemical reactions without being permanently
changed or depleted - Enzymes proteinaceous catalysts that increase
the rate of chemical reactions by lowering the
activation energy necessary for reaction to begin - Activation Energy minimum energy reactants must
have to start a chemical reaction
29
Activation Energy and Enzymes
30
Chemistry
- Inorganic Chemistry generally, substances that
do not contain carbon - Water, oxygen
- Exceptions CO, CO2, and HCO3-
- Organic Chemistry study of carbon-containing
substances. Those that are biologically active
are called biochemicals.
31
Water
- High specific heat large amount of heat required
to raise temperature of water - Stabilizes body temperature
- Protection
- Lubricant, cushion
- Participates in chemical reactions
- Many reactions take place in water
- Dehydration and hydrolysis
- Serves as a mixing medium
32
Mixtures, Solutions and Measures of Concentration
- Mixture substances physically but not chemically
combined - Suspension materials separate unless stirred.
Sand and water. - Colloid dispersal of tiny particles through a
medium. Milk. - Solution mixture of liquids, gasses, or solids
that are uniformly distributed and chemically
combined - Solvent that which dissolves the solute
- Solute that which dissolves in the solvent
- Concentration measure of number of particles of
solute per volume of solution - Unit used by physiologists is osmolality
- Concentration of body fluids influences movement
of fluid into and out of cells.
33
Acids and Bases Salts and Buffers
- Acid a proton donor or any substance that
releases hydrogen ions - Base a proton acceptor or any substance that
binds to or accepts hydrogen ions - Salt a compound consisting of a cation other
than a hydrogen ion and an anion other than a
hydroxide ion. Example NaCl - Buffer a solution of a conjugate acid-base pair
in which acid and base components occur in
similar concentrations
34
The pH Scale
- Refers to the Hydrogen ion concentration in a
solution - Neutral pH of 7 or equal hydrogen and hydroxide
ions - Acidic a greater concentration of hydrogen ions
- Alkaline or basic a greater concentration of
hydroxide ions
35
Oxygen and Carbon Dioxide Important Inorganic
Compounds
- Oxygen (O2) required in the final step in the
series of reactions used to extract energy from
food. - Carbon dioxide (CO2) produced during the
catabolism of organic compounds. - Metabolic waste product.
- Combines with water in plasma and forms H thus
affecting acid/base balance
36
Organic Chemistry Biochemicals
- Carbohydrates composed of carbon, hydrogen,
oxygen. - Divided into monosaccharides, disaccharides,
polysaccharides - Example glucose
- Energy sources and structure
- Lipids composed mostly of carbon, hydrogen,
oxygen. - Relatively insoluble in water.
- Example anabolic steroids
- Functions protection, insulation, physiological
regulation, component of cell membranes, energy
source - Proteins composed of carbon, hydrogen, oxygen,
nitrogen, sometimes iodine. - Example insulin
- Functions regulate processes, aid transport,
protection, muscle contraction, structure, energy - Nucleic Acids composed of carbon, hydrogen,
oxygen, nitrogen, phosphorus. - Examples ATP, DNA, RNA
37
Carbohydrates Monosaccharides
- Simple sugars.
- Six-carbon sugars like glucose, fructose, and
galactose are important in the diet as energy
sources. - Five-carbon sugars are components of ATP, DNA and
RNA
38
Carbohydrates Disaccharides
- Two simple sugars bound together by dehydration
- Examples sucrose, lactose, maltose
39
Macro molecules
- Carbohydrates
40
Carbohydrates Polysaccharides
- Long chains of many monosaccharides.
- Storage molecules for monosaccharides and form
part of cell surface markers - Glycogen formed by animals.
- Starch and cellulose formed by plants
- Starch in food is used as a source of
monosaccharides - Cellulose in food acts as fiber (bulk) in the
diet
41
Simple molecules
- Methane, Ethane, Propane, rings
42
Macro molecules
- Polymers
- Long chain lipids
- Carbohydrates
- Proteins
- Nucleic acids
43
Lipids Fats
- Ingested and broken down by hydrolysis
- Triglycerides composed of glycerol and fatty
acids - Functions protection, insulation, energy source
44
Macro molecules
- Long chain amphipatic molecules Phospholipids
45
Lipids Phospholipids
- Polar (hydrophilic) at one end nonpolar
(hydrophobic) at the other. - Function important structural component of cell
membranes
46
Lipids Steroids
- Cholesterol, bile salts, estrogen, testosterone.
- Carbon atoms arranged in four rings
- Functions physiological regulators and component
of cell membranes
47
Cell Membrane
- Double layer of phospholipids
48
Proteins
- Amino acids building blocks of protein
- Peptide bonds covalent bonds formed between
amino acids during protein synthesis
49
Protein Structure
- Primary sequence of amino acids in the
polypeptide chain - Secondary folding and bending of chain caused by
hydrogen bonding - Tertiary formation of helices or of pleated
sheets caused in part by S-S bonds between amino
acids - Quaternary two or more proteins associate as a
functional unit
50
Enzymes Protein Catalysts
- Lower the activation energy necessary for a
reaction to occur bring reactants into close
proximity - Three-dimensional shape contains an active site
where reactants attach. - Induced Fit Hypothesis enzymes change shape to
accommodate the shape of specific reactants - Enzyme names usually end in ase and often have
the same word stem as the reactant for example a
lipid is a reactant for lipase. - Cofactors combine with active site and make
nonfunctional enzymes functional - Organic cofactors called coenzymes
51
Nucleotides
- Composed of a five-carbon sugar, a nitrogenous
base, and a phosphate - Include the nucleic acids (DNA and RNA) and ATP
52
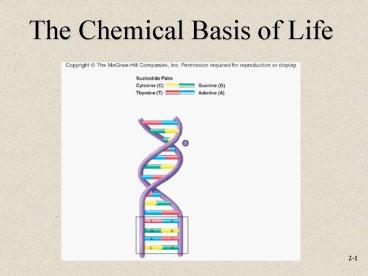
DNA Deoxyribonucleic acid
- Genetic material of cells copied from one
generation to next - Composed of 2 strands of nucleotides
- Each nucleotide contains one of the organic bases
of adenine or guanine (which are purines) and
thymine or cystosine (which are pyrimidines).
53
RNA Ribonucleic acid
- Similar to a single strand of DNA
- Four different nucleotides make up organic bases
except thymine is replaced with uracil
(pyrimidine) - Responsible for interpreting the code within DNA
into the primary structure of proteins.
54
Adenosine Triphosphate (ATP)
- Energy currency of the body
- Provides energy for other chemical reactions as
anabolism or drive cell processes as muscle
contraction - All energy-requiring chemical reactions stop when
there is inadequate ATP