active - PowerPoint PPT Presentation
1 / 15
Title:
active
Description:
They speed up the rate of chemical reactions without being consumed in the process. ... carbonic anhydrase - removes H2O from carbonic acid; amylase - digests starch; ... – PowerPoint PPT presentation
Number of Views:22
Avg rating:3.0/5.0
Title: active
1
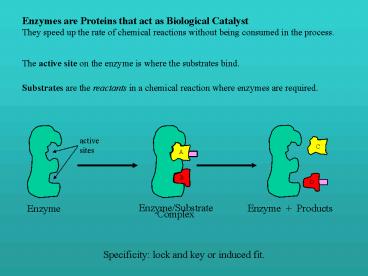
Enzymes are Proteins that act as Biological
Catalyst They speed up the rate of chemical
reactions without being consumed in the process.
The active site on the enzyme is where the
substrates bind.
Substrates are the reactants in a chemical
reaction where enzymes are required.
active sites
C
A
B
D
Enzyme
Enzyme/Substrate Complex
Enzyme Products
Specificity lock and key or induced fit.
2
(No Transcript)
3
Naming of Enzymes Most enzymes end with ase and
typically their name indicates their function or
the substrates they bind. Here are some examples
kinase - adds phosphates to molecules phosphotase
- removes phosphates from molecules
dehydrogenase - removes Hydrogens hydrolase -
adds H2O
synthase - dehydration synthesis reactions
carbonic anhydrase - removes H2O from carbonic
acid
amylase - digests starch lipase - digests
lipids protease - digests proteins.
4
Enzyme Specificity Specificity is ability of
enzyme to catalyze only certain reactions.
Some enzymes are very specific, such as
glucokinase. It catalyzes this reaction
glucose Pi glucose 6-P.
glucokinase
Other enzymes have broader specificities, e.g.
peptidases, which work on all peptide bonds.
The type and amount of enzymes in the body can
vary.
For example, alcohol dehydrogenase removes Hs
from ethanol (e.g., alcohol in wine) thereby
reducing its toxic effects.
Is everyones ability to handle alcohol the
same?
5
Enzyme Activation Some enzymes exists in an
inactive state and need to be activated to
catalyze.
1) Proteolytic activation
Pepsin (active)
Pepsinogen HCl (inactive)
2) Co-factors
Inorganic components required for substrate
binding at the active site.
e.g. Ca2, Mg2 or Cu2 (conformational changes).
3) Co-enzymes
Small organic molecules needed to accept and
transfer electrons (e-s) from different enzymatic
reactions.
e.g. NAD shuttles e-s in glycolysis.
6
Factors that Effect Enzyme Activity Once an
enzyme is active, several factors can modulate
(change) their activity.
1. pH (Acidity/Alkalinity) Enzymes function
within certain pH ranges. Changes in pH alter 3o
structure. Beyond a critical level (outside its
optimal pH range), the enzyme is denatured.
e.g., compare enzymes of mouth, stomach and small
intestine.
7
2. Temperature Enzymes function within certain
and temperature ranges, typically most enzymes in
the human body have an optimal level of activity
at around normal body temperature ( 36oC).
Again, beyond a critical level, enzymes are
denatured.
e.g., think of the effects of a fever on enzymes
in the body.
8
3. Chemical Modulators Chemical modulators are
molecules that bind to enzymes and alter
catalytic ability.
A) Competitive Inhibitors bind to the active site
without being acted on, thus reducing reaction
rate of true substrate(s). In other cases, the
competing molecule is acted on by the enzyme, but
again, inhibits reaction with natural substrate.
For example, ethylene glycol (antifreeze) is a
poison that can kill people. In the body,
ethylene glycol oxalic acid (toxic).
alcohol dehydrogenase
What is a treatment for ethylene glycol poisoning?
9
(No Transcript)
10
B) Non-Competitive Inhibitors bind at some site
other than the active site. They do not affect
enzyme-substrate binding but inhibit the enzyme
from catalyzing the reaction. Some act by binding
to the cofactors of enzymes.
11
4. Allosteric Modulators - these bind away from
active site but in doing so alter the shape of
the active site. This can increase or decrease
enzyme affinity.
Covalent Modulators - bind covalently to enzyme
away from the active site, change the shape, thus
function of the enzyme. e.g.,
Phosphate groups are one of the most common and
important covalent modulators in the human body.
active sites
active sites
Enzyme
12
Enzymes control metabolic pathways
e1
e3
e2
D
A
B
C
Substrate
Intermediates
End Product
Allosteric Inhibition substances bind away from
the active site, changing the shape of the enzyme
and thereby function of the enzyme.
In the case above, this can also be called End
Product Inhibition.
13
Enzyme and Substrate Concentration Affect the
Reaction Rate The rate of enzymatically
catalyzed reactions is assessed by measuring
product synthesis or substrate consumption.
Reaction Rate is
1. Directly Related to the Amount of Enzyme
Present. If the substrate concentration
(substrate) is kept constant, then the more
enzyme that is present, the greater the rate of
the reaction (i.e., the more product is
produced). See Graph
14
2. Related to the Amount of Substrate Present and
can Reach a Maximum. If the enzyme concentration
(enzyme) is held constant, the reaction rate
will increase as substrate increases but there
is a limit to how fast a reaction can go. See
Graph
15
(No Transcript)