Chapter - PowerPoint PPT Presentation
1 / 50
Title:
Chapter
Description:
Diagrams and Electron Dot Symbols - I ... Lewis Electron-Dot Symbols for Elements in ... Draw Lewis dot structures for the halogens. Try oxygen and nitrogen. ... – PowerPoint PPT presentation
Number of Views:27
Avg rating:3.0/5.0
Title: Chapter
1
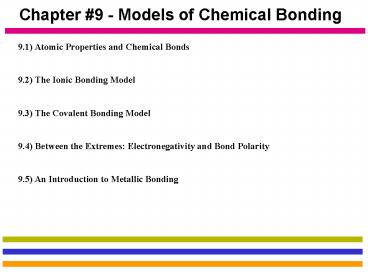
Chapter 9 - Models of Chemical Bonding
9.1) Atomic Properties and Chemical Bonds 9.2)
The Ionic Bonding Model 9.3) The Covalent
Bonding Model 9.4) Between the Extremes
Electronegativity and Bond Polarity 9.5) An
Introduction to Metallic Bonding
2
(No Transcript)
3
Sodium Chloride
4
Depicting Ion Formation with Orbital Diagrams and
Electron Dot Symbols - I
Problem Use orbital diagrams and Lewis
structures to show the formation of magnesium
and chloride ions from the atoms, and determine
the formula of the compound. Plan Draw the
orbital diagrams for Mg and Cl. To reach filled
outer levels Mg loses 2 electrons, and Cl will
gain 1 electron. Therefore we need two Cl atoms
for every Mg atom. Solution
Mg
Mg2 2 Cl-
..
2 Cl
.
..
..
..
.
Cl Cl
.
..
..
..
..
Mg
Mg2 2 Cl
.
..
..
5
Depicting Ion Formation from Orbital Diagrams and
Electron Dot Symbols - II
Problem Use Lewis structures and orbital
diagrams to show the formation of potassium and
sulfide ions from the atoms, and determine the
formula of the compound. Plan Draw orbital
diagrams for K and S. To reach filled outer
orbitals, sulfur must gain two electrons, and
potassium must lose one electron. Solution
2 K
2 K S - 2
S
..
.
.
2 -
.
..
..
..
..
..
K
.
S
2 K S
K
6
Three Ways of Showing the Formation ofLi and F
- through Electron Transfer
7
Lewis Electron-Dot Symbols for Elements in
Periods 2 3
8
The Reaction between Na and Br to Form NaBr
The Elements
The Reaction!
9
Vaporizing an Ionic Compound
10
Melting and Boiling Points of Some Ionic
Compounds
Compound mp( oC)
bp( oC)
CsBr 636
1300 NaI
661
1304 MgCl2
714 1412 KBr
734
1435 CaCl2
782
gt1600 NaCl 801
1413 LiF
845
1676 KF
858
1505 MgO 2852
3600
11
(No Transcript)
12
Figure 9.11 Potential-energy curve for H2.
13
Covalent Bonding in Hydrogen, H2
14
(No Transcript)
15
Figure 9.10 The electron probability
distribution for the H2 molecule.
16
Covalent bonds
http//wine1.sb.fsu.edu/chm1045/notes/Bonding/Cova
lent/Bond04.htm
animation
http//www.chem.ox.ac.uk/vrchemistry/electronsandb
onds/intro1.htm
17
For elements larger than Boron, atoms usually
react to develop octets by sharing electrons. H,
Li and Be strive to look like He. B is an
exception to the noble gas paradigm. Its happy
surrounded by 6 electrons so the compound BH3 is
stable. Try drawing a Lewis structure for
methane.
18
Draw Lewis dot structures for the halogens.
Notice that these all follow the octet rule!
Try oxygen and nitrogen.
These also follow the octet rule!
19
Bond Lengths and Covalent Radius
20
Figure 9.14 The HCl molecule.
21
The Charge Density of LiF
Fig. 9.20
22
Figure 9.12 Molecular model of nitro-glycerin.
What is the formula for this compound?
23
Rules for drawing Lewis structures
1. Count up all the valence electrons
2. Arrange the atoms in a skeleton
3. Have all atoms develop octets (except those
around He)
24
Make some Lewis Dot Structures with other
elements
SiH4
H2O
NH3
CH2O
C2H6
C2H6O
25
Figure 9.9 Model of CHI3Courtesy of Frank Cox.
CH3I
26
Make some Lewis Dot Structures with other
elements
CH4
H2O
NH3
CH2O
C2H6
C2H6O
27
Look at all these structures and make some
bonding rules
28
(No Transcript)
29
Rules for drawing Lewis structures
1. Count up all the valence electrons
2. Arrange the atoms in a skeleton
3. Have all atoms develop octets (except those
around He)
4. Satisfy bonding preferences!
30
A model of ethylene.
31
A model of acetylene.
32
A model of COCl2.
33
(No Transcript)
34
(No Transcript)
35
A model of SCl2.
36
Figure 9.16 Delocalized bonding in sodium metal.
37
Model of CO32-
38
(No Transcript)
39
(No Transcript)
40
The Relation of Bond Order,Bond Length and Bond
Energy
Bond Bond Order Average Bond
Average Bond
Length (pm)
Energy (kJ/mol)
C O 1
143 358 C
O 2
123 745 C O
3 113
1070 C C
1 154
347 C C 2
134
614 C C 3
121
839 N N 1
146
160 N N 2
122 418 N
N 3
110 945
Table 9.4
41
Conceptual Problem 9.103
42
Fig. 9.14
43
Figure 9.15 Electronegatives of the elements.
44
The Periodic Table of the Elements
2.1
He
0.9
1.5
2.0
2.5
3.0
3.5
4.0
Ne
Electronegativity
0.9
1.2
Ar
1.5
1.8
2.1
2.5
3.0
0.8
1.0
1.3
1.5
1.6
1.6
1.5
1.8
1.8
1.8
1.9
1.6
1.6
1.8
2.0
2.4
2.8
Kr
0.8
1.0
1.2
1.4
1.6
1.8
1.9
2.2
2.2
2.2
1.9
1.7
Xe
2.5
2.1
1.9
1.8
1.7
0.7
0.9
1.1
1.5
1.7
1.9
2.2
2.2
2.4
1.9
Rn
2.2
2.0
1.9
1.8
1.8
1.3
2.2
0.7
0.9
1.1
Ce Pr Nd Pm
Yb Lu
1.1
1.1
1.1
1.1
1.2
1.2
1.2
1.2
1.2
1.2
1.2
1.2
1.2
1.3
1.3
1.5
1.7
1.3
1.3
1.3
1.3 1.3
1.3
1.3
1.3
1.5
1.3
Th Pa U Np
No Lr
45
Fig. 9.16
46
Fig. 9.17
47
Determining Bond Polarity from
Electronegativity Values
Problem (a)Indicate the polarity of the
following bonds with a polarity arrow O -
H, O - Cl, C - N, P - N, N - S, C - Br, As - S
(b) rank those bonds in order of increasing
polarity. Plan (a) We use Fig. 9.16 to find the
EN values, and point the arrow toward the
negative end. (b) Use the EN values. Solution
a) the EN of O 3.5 and of H 2.1 O - H
the EN of O 3.5 and of Cl 3.0 O
- Cl the EN of C 2.5 and of P 2.1
C - P the EN of P
2.1 and of N 3.0 P - N the EN of N
3.0 and of S 2.1 N - S the
EN of C 2.5 and of Br 2.8
C - Br the EN of As 2.0 and of O
3.5 As - O
b) C - Br lt C - P lt O - Cl lt P - N lt N - S lt O -
H lt As - O 0.3 lt 0.4 lt 0.5 lt
0.9 lt 0.9 lt 1.4 lt 1.5
48
Fig. 9.18
49
Percent Ionic Character as a Function
ofElectronegativity Difference (?En)
Fig. 9.19
50
The Charge Density of LiF
Fig. 9.20