If we define Gi = G - PowerPoint PPT Presentation
Title:
If we define Gi = G
Description:
If we define Gi = G ( Pi = 1 atm ) then. Gf = G (T) RT ln Pf ... ( More chaos/randomness toward products ) H 0 larger Kp for more negative (large H ) H ... – PowerPoint PPT presentation
Number of Views:25
Avg rating:3.0/5.0
Title: If we define Gi = G
1
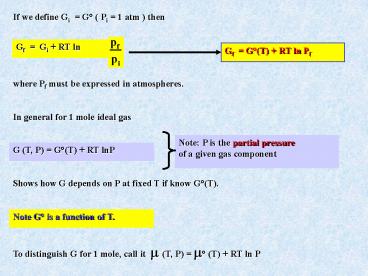
If we define Gi G ( Pi 1 atm ) then
Gf G(T) RT ln Pf
where Pf must be expressed in atmospheres.
In general for 1 mole ideal gas
Note P is the partial pressure of a given gas
component
G (T, P) G(T) RT lnP
Shows how G depends on P at fixed T if know G(T).
Note G is a function of T.
To distinguish G for 1 mole, call it ? (T, P)
? (T) RT ln P
2
? is free energy/mole for an ideal gas at T and
P. (Called chemical potential)
For n moles n? n? nRT ln P
Consider now the Chemical reaction
Free Energy for 1 mole of ideal gas D at a
partial pressure of PD
aA(PA) bB (PB) cC (PC) dD (PD)
?G ?Gprod - ?Greact
?G c?C RT ln PC d?D RT ln PD -
a?A RT ln PA - b?B RT ln PB
?G? c?C d?D - a?A - b?B ? ?G
3
?G ?G cRT ln PC dRT ln PD - aRT ln PA -
bRT ln PB
?G ?G RT ln (PC)c(PD )d/(PA)a(PB)b
True for arbitrary values of PC, PD, PA, PB
What happens if Pj happen to be those for
equilibrium?
Since initial and final states are in eq. ?G is
not neg for either direction. ? ?G 0 ?
Kp
?G - RT ln (PCeq)c (PDeq )d/(PAeq)a (PBeq)b
But Kp (PCeq)c (PDeq )d/(PAeq)a (PBeq)b!
4
?G -RT ln Kp
Remarkably important formula relates free energy
and Kp.
Since ?G is defined for a specific pressure of 1
atm, it is only a fct of T. Kp fct only of T!
Kp e -?G/RT 10 -?G/2.303RT
?G lt 0 ? exponent gt 0 Kp gt 1
?G gt 0 ? exponent lt 0 Kp lt 1
Reactions with a large neg ?G tend to proceed to
completion.
5
Bonus Bonus Bonus Bonus Bonus Bonus
Write ?G ?H - T?S ?
Kp e -?H/RT e ?S/R
Kp e -?G/RT
Kp 10 -?H/2.303RT10 ?S/2.303R
Larger ?S larger Kp. ( More chaos/randomness
toward products )
?H lt 0 ? larger Kp for more negative (large
??H?) ?H
Major T dependence for Kp is in ?H term
6
Remember old rule for shift in eq. with T.
Equilibrium shifts to left for an exothermic
reaction and to right for an endothermic
reaction.
Shift in Equilibrium with temperature
Kp e -?H/RT e ?S/R
If ?S roughly independent of T, then temperature
dependence of Kp is in e -?H/RT term.
?H lt 0 means heat released (exothermic) A ? B
heat
7
?H lt 0 means heat released (exothermic) A ? B
heat
Exponent -?H/RT gt 0 and increasing T causes
this to shrink so Kp gets smaller. Shift to left.
Think of heat as a reagent that works like common
ion effect A ? B heat
?H gt 0 heat absorbed (endothermic) heat A ? B
Kp e -?H/RT e ?S/R
When -?H /RT lt 0 look at e -?H/RT 1/
e?H/RT
Increasing T causes ?H/RT and hence e?H/RT to
get smaller.
Therefore, 1/ e?H/RT gets larger.
Kp increases and equilibrium shifts to right.
8
Connecting Kinetics and Equilibria
By definition, kinetic processes are not
equilibrium processes. In fact, we may think of
kinetic processes as the mechanism that nature
uses to reach the equlibrium state.
has 2 rate constants, we can write
kfAeBekrCeDe (Equilibrium
condition) Where Ae etc. are the equilibrium
concentrations of A etc.
kf/kr CeDe / AeBe Kequilibrium
9
Using the Arrhenius form for the rate constants
kf and kr
Keq kf/kr
(Af/Ar) exp -(EAf-EAr)/RT
But as we just learned (or you already knew from
high school)
lnKeq -?G0/RT
?G0 ?H0 - T ?S0
Where ?H0 is the enthalpy change for the
reaction and ?S0 is the entropy change for the
reaction. ?G0 is the Free Energy
But this gives Keq e -?G/RT e -?H/RT e
?S/R
10
Equating these two forms for the equilibrium
constant allows us to connect thermodynamics and
kinetics!
e (?S?/R) ? e (-?H?/RT)
(Af/Ar) exp -(EAf-EAr)/RT
Identify Af / Ar with e (?S?/R) (T
independent assuming ?Sº indep of T).
EAf - EAr identify with ?Hº
Activated State
EAf
EAr
A B
?Ho EAf - EAr
(?Ho Enthalpy change for AB ?CD)
C D