React with Metals Metal Reactivity - PowerPoint PPT Presentation
1 / 11
Title:
React with Metals Metal Reactivity
Description:
The more reactive the metal, the more vigorous the reaction. ... When metal carbonates react with acids they fizz giving off carbon dioxide gas. ... – PowerPoint PPT presentation
Number of Views:172
Avg rating:3.0/5.0
Title: React with Metals Metal Reactivity
1
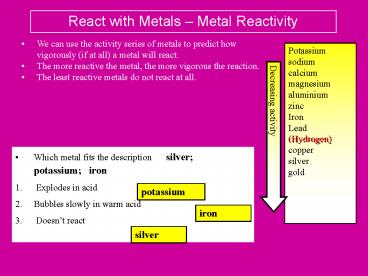
React with Metals Metal Reactivity
- We can use the activity series of metals to
predict how vigorously (if at all) a metal will
react. - The more reactive the metal, the more vigorous
the reaction. - The least reactive metals do not react at all.
- Which metal fits the description silver
potassium iron - Explodes in acid
- Bubbles slowly in warm acid
- Doesnt react
potassium
iron
silver
2
Reaction with Metals - general
- Many metals react with acids to form a salt and
hydrogen gas. - The general word equation is
- The salt will depend upon the metal and the acid
used.
- Hydrochloric acid gives metal chlorides
- Sulphuric acid gives metal sulphates
- Nitric acid gives metal nitrates
- So, for example
hydrogen
magnesium chloride
?
hydrochloricacid
magnesium
3
Evidence of Reaction
Some of these reactions show bubbles being
produced. These are bubbles of hydrogen gas. This
proves that a reaction is taking place because a
new substance is forming. How do we test for
hydrogen?
Oxygen in the air
hydrogen
Mg acid
4
Formula of salts
- Chemists often use formula equations to show not
just what atoms are present but also how many. - To do this we need to understand what the numbers
in a formula represent. Here are some examples.
Name of Salt
Contains
Formula
sodium chloride
one Cl
one Na
NaCl
magnesium chloride
two Cl
one Mg
MgCl2
potassium nitrate
three O
one N
one K
KNO3
potassium sulphate
four O
one S
two K
K2SO4
calcium nitrate
six O
two N
one Ca
Ca(NO3)2
5
Activity
- How many of each type of atom does the formula of
the salt represent?
one
two
two
one
four
one
one
four
two
one
six
one
one
four
6
Formula Equations
- Step 1 Write down the word equation.
- Step 2 Replace words with the chemical formula .
(You will be given or need to look up the formula
at this stage.) - Step 3 Check that there are equal numbers of
each type of atom on both sides of the equation.
If not, then balance the equation by using more
than one. - E.g. The reaction of magnesium with hydrochloric
acid
Neither H nor Cl balance.Need 2 HCl
7
Activity
- Can you finish off the equations below
- Step 1 Write down the word equation.
- Step 2 Replace words with the chemical
formula. - Step 3 Balance the type of atom on both sides.
Already balances No change
Need 2 K on both sides
8
Metal oxides and acids
Metal oxide acid ? a salt water
- Most metal oxides are not soluble.
water
copper sulphate
9
Metal oxides and acids
Metal oxide acid ? a salt water
water
zinc sulphate
water
sodium chloride
water
iron nitrate
10
Metal carbonates and acids
Metal carbonate acid ? a salt
water carbon dioxide
- When metal carbonates react with acids they fizz
giving off carbon dioxide gas. - Most metal carbonates are not very soluble and so
reactions may be slow.
water
calcium chloride
carbon dioxide
11
Metal carbonates and acids
Metal carbonate acid ? a salt water
carbon dioxide
water
zinc sulphate
?
sulphuric acid
carbon dioxide
water
sodium chloride
?
hydrochloric acid
carbon dioxide
water
nickel nitrate
?
nitric acid
carbon dioxide