Applications of Solubility - PowerPoint PPT Presentation
Applications of Solubility
To 'soften' water, washing soda may be added (Na2CO3) The washing soda precipitates the metal ions: Ca2 (aq) CO32-(aq) CaCO3(s) ... – PowerPoint PPT presentation
Title: Applications of Solubility
1
- Applications of Solubility
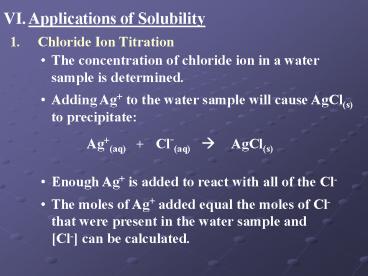
1.
Chloride Ion Titration
- The concentration of chloride ion in a water
sample is determined. - Adding Ag to the water sample will cause AgCl(s)
to precipitate
Ag(aq) Cl-(aq) ? AgCl(s)
- Enough Ag is added to react with all of the Cl-
- The moles of Ag added equal the moles of Cl-
that were present in the water sample and Cl-
can be calculated.
2
eg
25.0mL of a water sample was titrated with 0.500M
AgNO3(aq), using sodium chromate as an indicator.
At the equivalence point 26.8mL of AgNO3
solution had been added. What was the Cl- in
the water sample?
- Moles of AgNO3 used
Ag(aq) Cl-(aq) ? AgCl(s)
- moles of Cl- moles of AgNO3 used
3
2.
Softening Hard Water
- Hard water is caused by the presence of metal
ions such as Ca2 and Mg2 - These ions react with soap molecules and inhibit
their function. - Slow-moving ground water (such as well water) is
most likely to be hard (more time for ions to
dissolve into the water). - To soften water, washing soda may be added
(Na2CO3) - The washing soda precipitates the metal ions
Ca2(aq) CO32-(aq) ? CaCO3(s)
Mg2(aq) CO32-(aq) ? MgCO3(s)
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.