Spectrochemical methods of Analysis - PowerPoint PPT Presentation
1 / 18
Title:
Spectrochemical methods of Analysis
Description:
... of the spectrophotometer (see light diagram for spectrophotometer) ... reflected from surfaces within the spectrophotometer that reaches the detector. ... – PowerPoint PPT presentation
Number of Views:293
Avg rating:3.0/5.0
Title: Spectrochemical methods of Analysis
1
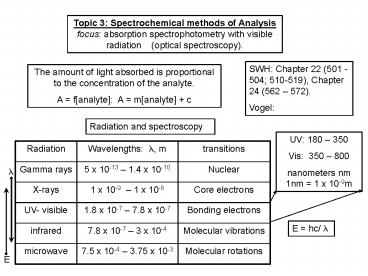
Topic 3 Spectrochemical methods of Analysis
focus absorption spectrophotometry with visible
radiation (optical spectroscopy).
SWH Chapter 22 (501 -504 510-519), Chapter 24
(562 572). Vogel
The amount of light absorbed is proportional to
the concentration of the analyte. A fanalyte
A manalyte c
Radiation and spectroscopy
UV 180 350 Vis 350 800 nanometers nm 1nm
1 x 10-9m
?
E hc/ ?
E
2
Topic 3 Spectrochemical methods of Analysis
focus absorption spectrophotometry with visible
radiation (optical spectroscopy) continued
3.1 Instruments
P0(?)
P(?)
E
Detector readout
Wavelength selector
Source
sample
Cells Quartz 180 3500 nm Si glass 380 2000
nm
W 350 -2200 nm D2 160 380 nm
SWH p 564
3
Topic 3 Spectrochemical methods of Analysis
focus absorption spectrophotometry with visible
radiation (optical spectroscopy) continued
SWH p 504
3.2 Absorption Spectra
T, called the transmittance, Is a measure of the
light that passes through the sample T P/Po
Samp l e
Po P
A, the absorbance, is defined by A
log10(Po/P) -log10(T)
? - the Cell Path length
Energy is absorbed through electronic transitions
within the bonding orbitals of the analyte Eg
Sites of unsaturation in organic molecules eg
double bonds (-CC- -CO, -NCO) ?? ? , or d
?d transitions in metal complexes or Metal d ?
ligand transitions
?max wavelength at which A is a maximum
KMnO4
4
3.3 The Beer-Lambert Law
A ? c ?
?
Lamberts Law A ? ?
Beers law A ? c
c - concentration
- the proportionality constant, is called the
extinction coefficient or molar absorbtivity. - is the slope of the plot of A against c.
- The magnitude of ? is wavelength dependent.
Units of ? concentration-1.length-1 eg dm-3
mol-1 cm-1
5
Topic 3 Spectrochemical methods of Analysis
focus absorption spectrophotometry with visible
radiation (optical spectroscopy) continued
3.4 Analyses by Spectrophotometric methods.
- Qualitative A ? c ?
- Many organic functional groups have well defined
absorption characteristics ?max ?
(usually in the UV) - - prepare a solution of known concentration (c)
- - record the spectrum between 180 and 350nm
- - identify ?max, calculate ? consult texts.
Transition metals often coloured due to
transitions between d orbitals absorb in the
visible region, the number of peaks and the ?max
? identify the metal ion geometry - Oh vs
Td
SWH p560
Non-coloured analytes can react to give coloured
compounds eg PO43-
6
3.4 Analyses by Spectrophotometric methods
continued.
- b) Quantitative analyses
- i) Use the Beer-Lambert equation A ? c ?
- prepare a solution of analyte and add reagents
- measure the absorbance at known ?max and ?
- look up ? and calculate c.
- Molar absorptivities are not very precisely know,
amongst other things (see later), and so such
calculations are not very precise. - Seldom done this way
- ii) Use calibration curves the common method.
- Prepare a set of standards
- Measure their absorbances at ?max
- Plot A against c
- A manalytestandards c
- Measure the analyte absorbance
- Calculate analyte concentration
- analytesample (A c)/m
7
3.4 Analyses by Spectrophotometric methods
continued.
3.4.1 The determination of PO43- in aqueous
samples
Main species in aqueous solutions in the pH 3
11 range HPO42- H2PO4- Colourless species
Phosphoric acid H3PO4 H2O ? H2PO4- H3O
pKa1 2.12 H2PO4- H2O ? HPO42- H3O
pKa2 7.21 HPO42- H2O ? PO43- H3O
pKa3 12.3.
The analysis is based on the reaction of PO43-
with molybdate H2PO4- 12HMoO3 14H2O ?
P(Mo3O10)43- 14H3O. if pH 7.2 a deep yellow
colour (?max 420nm).
P(V) in a tetrahedral array of Mo3O102-
units. Cluster formation is pH dependent
structure and rate.
In the presence of potassium antimonyl tartrate
(KSbC4H4O7) and at pH lt 1 ascorbic acid will
reduce some of the Mo(VI) centres to Mo(V) to
give a mixed oxidation state cluster of intense
blue colour. (Mo blue) ?max 880nm, ?
23,000 dm3 mol-1 cm-1.
8
3.4.1 The determination of PO43- in aqueous
samples continued
The analytical method from Standard Methods
for the Examination of Water and Wastewater.
Validated but must be verified within your
laboratory
sample
The various treatments allow for the speciation
of the P in the sample
Total P
Filter (0.45 µm)
Suspended matter
filtrate
colourimetry
Soluble reactive phosphorus (SRP) (
orthophosphate)
H2SO4 hydrolysis colourimetry
S2O86- digest colourimetry
Soluble acid-hydrolysable P SRP
Total dissolved P
9
3.4.1 The determination of PO43- in aqueous
samples continued
The analytical method from Standard Methods
for the Examination of Water and Wastewater.
4500-P E. Ascorbic Acid Method
Units mgP/L ? mgPO43-/L but mM P mM
PO43- eg 2mgP/L 2/31 mM while 2mgPO43-/L
2/95mM
- Principle Mo-blue via ascorbic acid reduction.
- Interferences
- AsO43- at gt 0.1mg As/L
- Cr(VI) at gt 1mgCr/L
- NO2- at gt 1mg N/L
- S2- lt 1mgS/L SiO44- lt 10mgS/L do not interfere
- Detection limit 10µgP/L
- 0.3 2 mgP/L ? 0.5cm
- 0.15 1.3 mgP/L ? 1.0cm
- 0.01 0.25 mgP/L ? 5.0cm
10µP/L 0.3µM 2mgP/L 65µM
PO43- in tropical coastal waters gt 0.1 µM
considered to be detrimental to corals
10
3.4.1 The determination of PO43- in aqueous
samples continued
- d) Reagents
- 5N H2SO4 70 mL conc. H2SO4 to 500 mL distilled
water (DW). - Potassium antimonyl tartrate 1.3715g
K(SbO)C4H4O6. ½ H2O in 500 mL DW. - Ammonium molybdate 20 g (NH4)6Mo7O24.4H2O in
500 mL DW. - Ascorbic acid 1.76 g ascorbic acid in 100 mL
DW. - Combined reagent
- 50 mL a) 5 mL b) 15 mL c) 30 mL d)
- in that order with mixing after each addition.
Full details of preparations given in Standard
Method reference. Storage conditions, shelf lives
also given. 1 week at 4C 4 hours
Standards Stock 219.5 mg anhydrous KH2PO4 in
1000 mL DW 1.00 mL 50.0 µg PO43- - P. Standard
phosphate solution 50 mL stock to 1000 mL
DW 1.00 mL 2.5 µg P
Units 1.00 mL 50.0 µg PO43- - P
???. 219.5/(39.1 2 31 64)/L 1.613
mM 1.613 mM 1.613 x 31 mgP/L 50.0 mgP/L 1.613
mM 1.613 x 95 mgPO43-/L 153 mgPO43-/L
50.0 µg PO43- - P means 50.0 µg P present as
PO43-.
11
3.4.1 The determination of PO43- in aqueous
samples continued
e) Procedure Sample add 50 mL sample to a 125
mL erlenmeyer flask and add 1 drop
phenolphthalein indicator. If red add 5N H2SO4
dropwise until just colourless. Add 8 mL mixed
reagent, wait at least 10 minutes but no more
than 30 minutes, Measure absorbance at 880nm
using 0 mgP/L standard as reference solution.
Calibration standards treat as for sample.
Calibration curve 6 standards plus blank over
range required 3 ranges 0.3 2 mgP/L
? 0.5 cm 0.15 1.3 mgP/L ? 1.0
cm 0.01 0.25 mgP/L ? 5.0 cm 50 mL
standard - 2.5 mgP/L 40 mL
standard to 50 mL 2.0 mgP/L 30 mL standard to
50 mL 1.5 mgP/L 20 mL standard to 50 mL 1.0
mgP/L 10 mL standard to 50 mL 0.5 mgP/L 5 mL
standard to 50 mL 0.25 mgP/L 0 mL standard to
50 mL 0.0 mgP/L For 2nd range dilute standard
by 2 and then as above. For 3rd range dilute
standard by 10 and then as above. Prepared daily.
Follow procedure carefully, use clean glassware,
proper pipette and volumetric flask techniques
a good calibration curve suggests adequate care.
12
3.4.1 The determination of PO43- in aqueous
samples continued
- Preparation of standards
- Weigh accurately about 0.13 g KH2PO4 and
dissolve in 1000 mL DW. 30 mgP/L. Stock A - 5 mL to 250 mL 600 µgP/L. Stock B
- 25 mL to 500 mL 30 µgP/L. Stock C
Application of the method to marine waters. From
a detection limit of 1 µgP/L ( 0.03 µM) to about
30 µgP/L ( 1 µM). How should the standards be
prepared?
Need six standards 0 30 µgP/L ? 0, 6, 12, 18,
24, 30 30 µgP/L 30/31 x 136.1 µgKH2PO4/L
132 µgKH2PO4/L Need to weigh at least 100 mg
KH2PO4 to minimize weighing errors. If we weigh
132 mg KH2PO4 and dissolve that in 1000 mL the 1
in 1000 dilution will give us our maximum
standard Small pipettes can lead to large errors
do the 1000 fold in 2 steps a 50 then 20 fold
dilutions
32.69 26.15 19.61 13.07 6.54
If actual weight 0.1435g KH2PO4 to 1 L then
stock A 23.69 mgP/L and stock B 653.7
µgP/L and stock C 32.69 µgP/L
13
3.4.1 The determination of PO43- in aqueous
samples continued
Develop the colour, wait 10 15 minutes,
measure absorbance at 880 nm
Abs 0.00376 µgP/L 3x10-5.
- Plot the actual standard concentrations
- Points dont fall exactly on the best fit line
experimental uncertainties - Slope ? 0.00376 (µgP/L)-1 (5 cm)-1 0.000752
(µgP/L)-1 (cm)-1 - 0.000752x31 (µmol/L)-1 cm-1. 0.0233
(µmol/L)-1 cm-1. - or ? 23,300 (mol/L)-1 cm-1.
- PO43-sample (Asample - 3x10-5)/0.00376 or
read from graph
14
Topic 3 Spectrochemical methods of Analysis
focus absorption spectrophotometry with visible
radiation (optical spectroscopy) continued
3.5 Deviations from the Beer-Lambert Law.
Primarily due to the limitations of Beers Law
A ? c
- Effect of high concentrations
- high analyte concentrations gt 0.01M, or
- the analyte in high electrolyte concentrations.
- Arise from interactions between species in
solution which become significant at high
concentrations (ions get close
together). - Lead to curvature of the calibration line
- i.e. ?, the molar absorptivity changes with
concentration. - Can no longer assume linearity c ? A/???
- Chemical Effects
- ?? is species specific, and thus analytical
conditions must be such that all the analyte is
present as one species - eg. Cr2O72- 2H2O ? 2CrO42- 2H3O
- for Cr2O72- ?max 450nm while for CrO42- ?max
368nm - A change in the pH changes the species
distribution.
Analytical procedures must be adhered to.
15
3.5 Deviations from the Beer-Lambert Law
continued.
c) Instrumental Effects Beers Law only strictly
applies to monochromatic light (one ? only) but
monochromators select a small range of
wavelengths around the wavelength of choice.
Consider a situation where the wave-package from
the monochromator ranged between ?1 and ?2 The
light passing through the sample will be the sum
of all the wavelengths from ?1 to ?2.
?2
?1
- Pt P(1) P(2)
- As log(Po(1) Po(2))/(P(1) P(2))
- (Po(1) Po(2))
- Po(1)10-??1c? Po(2)10-??2c?
Just considering the two extremes A1 ??1c?
and A2 ??2c? By definition A1
logPo(1)/P(1) ? 10A1 Po(1)/P(1) i.e. P(1)
Po(1)/10A1 or P(1) Po(1)10- ? ?1c? Similarly
P(2) Po(2)10-??2c?
log
16
3.5 Deviations from the Beer-Lambert Law
continued.
- (Po(1) Po(2))
- Po(1)10-??1c? Po(2)10-??2c?
As log
Assume Po(1) Po(2) for simplicity. then if ??1
??2 As log2Po(1)/(2Po(1)10-??1c?)
log(10??1c?) ??1c?
?2
?1
But if ??1 2??2 and A1 1, for
example As log2Po(1)/Po(1)(10-??1c?
10-1/2??1c?) log2/101 101/2
log2/0.1 .316 0.69 etc. Normally
not a major problem provided the absorbance does
not show large changes with wavelength.
Abs
Conc.
17
3.5 Deviations from the Beer-Lambert Law
continued.
c) Instrumental effects continued Band widths
controlled by the monochromator and light-pass
slits between the components of the
spectrophotometer (see light diagram for
spectrophotometer). Stray light light reflected
from surfaces within the spectrophotometer that
reaches the detector. Normally also measured
with the blank (reference solution) and therefore
of minimal effect.
- Applications
- Clinical, inorganic, organic, biochemical
applications. - Very high sensitivity normally analyte lt 0.01
M. c.f. titrations 0.1 M - Low detection limits.
- Reasonable selectivity few interferences in
most methods. - Good precision 5 of calculated
concentrations. - Convenient rapidly performed, minimal costs
- single beam, visible US1000 through
double beam, UV/vis, US5000.
18
- Problems
- SWH chapter 22 22.2 (d, i, j, k, l, m).
22.7, 22.11, 22.14. - - chapter 24 24.1 (b), 24.4, 24.6, 24.27 (a)
and if absorbance of a sample was 0.427 calculate
the concentration of Fe in the sample. And - 1. Nitrate is to be determined in an aqueous
sample. The concentration is expected to be
between 20 and 80 µM. Give details of how you
would prepare a set of suitable standards from
primary standard KNO3. - From the calibration curve in problem 24.27 above
calculate the molar absorptivity. - Past exam paper1999, paper 1, section B. 2000,
paper 1, section B (except 4 (a),). 2001, paper
1, section B (except 3 (b), 4 (a)). 2002,
section B. 2003 ??
anthony.greenaway_at_uwimona.edu.jm Mondays C 26Q
laboratory, second floor of new chemistry
building. 10.00am 5.00pm.