Polyprotic Acids
1 / 27
Title: Polyprotic Acids
1
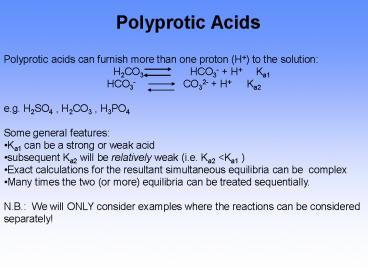
Polyprotic Acids
Polyprotic acids can furnish more than one
proton (H) to the solution
H2CO3 HCO3-
H Ka1 HCO3- CO32- H Ka2 e.g.
H2SO4 , H2CO3 , H3PO4 Some general
features Ka1 can be a strong or weak
acid subsequent Ka2 will be relatively weak
(i.e. Ka2 ltKa1 ) Exact calculations for the
resultant simultaneous equilibria can be complex
Many times the two (or more) equilibria can be
treated sequentially. N.B. We will ONLY
consider examples where the reactions can be
considered separately!
2
Polyprotic Acids
Phosphoric acid A triprotic acid.
H3PO4 H2O H3O H2PO4-
Ka 7.1x10-3
H2PO4- H2O H3O HPO42-
Ka 6.3x10-8
HPO42- H2O H3O PO43-
Ka 4.2x10-13
3
Phosphoric Acid
- Ka1 gtgt Ka2
- All H3O is formed in the first ionization step.
- H2PO4- essentially does not ionize further.
- Assume H2PO4- H3O.
4
Polyprotic Acids - An Example
Consider the dissociation of H2SO3 (sulfurous
acid) H2SO3 H HSO3- Ka1 1.2x10-2
HSO3- 2H SO32- Ka2 6.2x10-8 This acid
provides two equivalents of protons per
equivalent of acid. We can consider the first
reaction to be 100 complete before we consider
the second reaction to occur at all. Lets look
at this mathematically
5
First Dissociation
Calculate the pH of a 0.45 M solution of
H2SO3. H2SO3 H HSO3- 2H SO32-
Ka1 1.2x10-2 Ka2 6.2x10-8
H2SO3 H HSO3- Initial
0.45 -- -- Change - x x x Equilibrium
0.45 - x x x
x 0.072 M H HSO3-
6
First Dissociation
Calculate the pH of a 0.45 M solution of
H2SO3. H2SO3 H HSO3- 2H SO32-
Ka1 1.2x10-2 Ka2 6.2x10-8
H2SO3 H HSO3- Initial 0.45 -- -- Change
- 0.072 0.072 0.072 Equilibrium 0.38
0.072 0.072 The contribution from the first
equilibrium is H 0.072 M
7
Second Dissociation
Calculate the pH of a 0.45 M solution of
H2SO3. H2SO3 H HSO3- 2H SO32-
Ka1 1.2x10-2 Ka2
6.2x10-8
HSO3-
H
SO32- Initial 0.072 0.072 Change - x x
x Equilibrium 0.072 -x 0.072x x
x 6.2x10-8 M
8
Second Dissociation
Calculate the pH of a 0.45 M solution of
H2SO3. H2SO3 H HSO3- 2H SO32-
Ka1 1.2x10-2 Ka2 6.2x10-8
HSO3-
H SO32- Initial 0.072
0.072 Change 6.2x10-8 6.2x10-8 6.2x10-8
Equilibrium 0.072 0.072 6.2x10-8
H 0.072 M pH -log H 1.2
9
A Question from Test 2 2004
- (c) Sulfur dioxide (SO2) will react with water to
generate sulfurous acid, H2SO3. Calculate the pH
of a 10.00M solution of this acid. Ka1 1.3 x
10-2 Ka2 6.2 x 10-8
H2SO3 H HSO3- 2H SO32- Ka1
1.2x10-2 Ka2 6.2x10-8
H2SO3 H HSO3- Initial 10 -- -- Change - x
x x Equilibrium 10 - x x x
x can be neglected
H (1.3 x 10-2)(10M)1/2 0.36 M (3.6
dissociated) (solved with quadratic x 0.36M)
pH 0.439 0.44
10
Ions as Acids and Bases
CH3CO2- H2O CH3CO2H OH-
base
acid
NH4 H2O NH3 H3O
base
acid
Ka Kb Kw
This is an important general relationship for any
acid and its conjugate base!
11
Hydrolysis
- Water (hydro) causing cleavage (lysis) of a bond.
Na H2O ? Na H2O
No reaction
No reaction
Cl- H2O ? Cl- H2O
NH4 H2O ? NH3 H3O
Hydrolysis
12
Acid-Base Properties of Salts
13
Acid-Base Properties of Salts
MX H2O acidic or basic solution? Consider
ammonium chloride NH4Cl(aq) ? NH4(aq) Cl-
(aq) (a) Reaction of Cl- with H2O
Cl- H2O ? HCl OH-base acid
acid base
Cl- is a VERY weak base because its conjugate
acid is strong. Therefore, Cl- makes a neutral
solution.
14
Acid-Base Properties of Salts
NH4Cl(aq) ? NH4(aq) Cl- (aq)
(b) Reaction of NH4 with H2O
NH4 H2O ? NH3 H3Oacid
base base acid
NH4 is a moderate acid because its conjugate
base is weak. Therefore, NH4 makes an acidic
solution
15
Acid-Base Properties of Salts
Calculate the pH of a 0.10 M solution of
Na2CO3 Na H2O ? neutral CO32- H2O ? HCO3-
OH-base acid acid base Notice that this is
a POLYPROTIC base CO32- H2O ? HCO3-
OH-HCO3- H2O ? H2CO3 OH- We treat this the
same way we treat a polyprotic acid. We consider
the equilibria to be separated enough to allow us
to consider them to be occurring
sequentially. For the above example, we only
consider the first protonation.
16
Acid-Base Properties of Salts
CO32- H2O ? HCO3- OH- base acid acid
base
Kb 2.1 x 10-4
Initial 0.10 change -x x x equilib 0.10 -
x x x
17
Acid-Base Properties of Salts
Make the assumption x ltlt CO3-2 x
(2.1x10-4 )(0.10)1/2 0.0046
H2O CO32- ? HCO3- OH- Initial
0.10 change - 0.0046 0.0046
0.0046 equilib 0.10 0.0046 0.0046 pOH
- log OH- -log(0.0046) 2.34 pH pOH
14 so pH 11.66
18
A Question from Test 2 2004 Hydrolysis of the
Conjugate Base of a Weak Acid
- Calculate the pH of a 0.1M solution of NaOBz.
The Ka of HOBz 6.6 x 10-4 - Which of the following is the balance reaction
that we should consider??
(a) HOBz H2O ? OBz- H3O (b) OBz- H2O ?
HOBz OH- (c) 2 H2O ? H3O OH- (d) HOBz
OH- ? OBz- H2O
19
A Question from Test 2 2004 Hydrolysis of the
Conjugate Base of a Weak Acid
- In class, we learned that the conjugate base of a
weak acid can react with water via the hydrolysis
reaction. Calculate the pH of a 0.1M solution of
NaOBz. The Ka of HOBz 6.6 x 10-4
The reaction of interest is the hydrolysis of
OBz- OBz- H2O ? HOBz OH- (our solution
should be basic!) We will need the Kb for the
OBz- anion. This is given by the relationship
KbKa Kw
20
A Question from Test 2 2004 Hydrolysis of the
Conjugate Base of a Weak Acid
- In class, we learned that the conjugate base of a
weak acid can react with water via the hydrolysis
reaction. Calculate the pH of a 0.1M solution of
NaOBz. The Ka of HOBz 6.6 x 10-4
The reaction of interest is the hydrolysis of
OBz- OBz- H2O ? HOBz OH- (our solution
should be basic!) We will need the Kb for the
OBz- anion. This is given by the relationship Kb
HOBzOH-/OBz- Kw/Ka 1.515 x 10-11
Now set up the ICE table OBz- HOBz
OH- I 0.1 C -x x x E 0.1 -x x x
x2/(0.1-x) 1.515 x 10-11 using the assumption
that xltlt 0.1 x 1.231x 10-6 OH- pOH 5.91
and pH 8.09
21
Molecular Structure and Acid-Base Behavior
- Why is HCl a strong acid, but HF is a weak one?
- Why is CH3CO2H a stronger acid than CH3CH2OH?
- There is a relationship between molecular
structure and acid strength. - Bond dissociation energies are measured in the
gas phase and not in solution.
22
Strengths of Binary Acids
HI HBr HCl HF
160.9 gt 141.4 gt 127.4 gt 91.7 pm
Bond length
297 lt 368 lt 431 lt 569 kJ/mol
Bond energy
109 gt 108 gt 1.3x106 gtgt 6.6x10-4
Acid strength
HF H2O ? F-H3O F- H3O
free ions
ion pair H-bonding
23
Strengths of Oxoacids
- Factors promoting electron withdrawal from the OH
bond to the oxygen atom - High electronegativity (EN) of the central atom.
- A large number of terminal O atoms in the
molecule.
H-O-Cl H-O-Br ENCl 3.0 ENBr 2.8 Ka
2.9x10-8 Ka 2.1x10-9
24
Strengths of Oxoacids
Ka large! Ka 1.3x10-2
25
Strengths of Organic Acids
O
H
H
H
C
C
O
C
H
H
O
C
H
H
H
H
H
acetic acid ethanol
Ka 1.8x10-5 Ka 1.3x10-16
26
Structural Effects
H
O
Ka 1.8x10-5 Ka 1.4x10-3
C
C
H
-
O
H
Cl
O
C
C
H
-
O
H
27
Lewis Acids and Bases
- Lewis Acid
- A species (atom, ion or molecule) that is an
electron pair acceptor. - Lewis Base
- A species that is an electron pair donor.
adduct
base
acid