NGFR p75 - PowerPoint PPT Presentation
1 / 14
Title:
NGFR p75
Description:
Presentation of pathogen Ags to na ve T cells by the APC induces IL-12Rb2 ... Immunogenic DCs stimulate and bias na ve CD4 T cells into TH1/TH2 cells and ... – PowerPoint PPT presentation
Number of Views:43
Avg rating:3.0/5.0
Title: NGFR p75
1
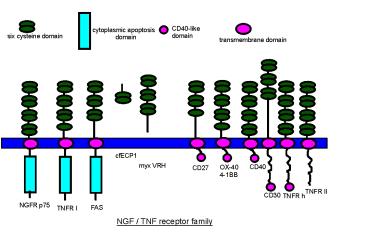
CD40-like
cytoplasmic apoptosis
domain
six cysteine domain
domain
transmembrane domain
cfECP1
myx VRH
CD40
OX-40
CD27
4-1BB
TNFR II
CD30
TNFR h
NGFR p75
FAS
TNFR I
NGF / TNF receptor family
2
(No Transcript)
3
(No Transcript)
4
CYTOKINES IN THE ACTIVATION OF T cells
5
virus
macrophage / dendritic cell
mast cell
TLR
APC
Ag
Costimulatory molecules
MHC Class II
MHC Class I
(I) CD80 / B7.1 (ii) CD86 / B7.2 (iii) CD40
IL-2
g
IFN-
CD28
TCR
IL-12Rß2
IFN-
R
IL-2R
g
Tc
IL-2R
IL-4R
THP/TH0
CD8
IL-18R
IL-2
CD4
IL-12 IL-18
IL-4
TH2
TH1
g
IFN-
TNF
CD4
CD4
IL-10
PROLIFERATION
PROLIFERATION
TNF
6
T cell lineage commitment
- The APC is activated by the pathogen, which may
be an intracellular organism. surface TLRs
recognize the pathogen. TLR activation by
pathogen results in e.g. IL-12 production. - Presentation of pathogen Ags to naïve T cells by
the APC induces IL-12Rb2 expression on T cells. - APC-derived IL-12 initiates TH1 development.
IL-12 acts via STAT4 to upregulate IFN-g
production. - TH1 development requires IFN-g itself, which
activates STAT1 and induces transcription of the
transcription factor T-bet. T-bet is a major TH1
commitment factor and transactivates the IFN-g
gene, as well as inducing chromatin remodelling
of the IFN-g gene locus. - IL-12 induces IL-18R expression, which allows
IL-18 to synergize with IL-12 to increase IFN-g
production from committed TH1 cells. - NET RESULT much IFN-g is produced, which
negatively regulates commitment to the TH2
lineage. - This sequence of events occurs if there is
abundant pathogen and if the intracellular
pathogen, when processed, presents Ag in the
context of MHC Class II such that there is a high
affinity event with the TCR on the naïve T cell. - A low affinity event results in lineage
commitment to TH2, and IL-4 and IL-10 production.
Hellminths and allergens promote TH2 lineage
cell commitment. Not clear whether NK T cells,
mast cells or differentiating T cells are the
major source of IL-4. IL-10 is a negative
regulator of TH1 lineage commitment. - Also note that engagement of the TCR by Ag
presented by MHC results in engagement of a
number of costimulatory molecules between the APC
and the naïve T cell, enhancing the activation of
the T cell. - IL-1 also plays an important role in the
activation of the naïve T cell.
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
T cell Lineage
Th1
Th2
Treg
IFN-? producing -Intracellular pathogens
IL-4, IL-5 producing-Parasitic infection
Suppression regulation
11
Diversification of CD4 T Cell Lineages
Although functional CD4 T cell development has
been dominated by the Th1-Th2 paradigm for nearly
two decades, the number of defined lineages has
now increased. The cytokines associated with
arrows indicate dominant cytokines involved in
specification of each of the indicated lineages.
The cytokines listed below each cell type
indicate key effector or regulatory cytokines
produced by differentiated cells of that lineage
or, in the case of nTreg, a contact-dependent
mechanism of suppression. Tn naive, postthymic
CD4 T cell precursors Tp thymic precursors.
Dotted lines represent less well-defined lineage
relationships.
12
Model of Branching Th17 and Adaptive Treg Lineage
Development
This model emphasizes distinct pathways leading
to mature Th17 effector cells or Foxp3 adaptive
Tregs (aTreg), induced by a common requirement
for TGF-ß but differential effects of IL-6 and
IL-23. Naive CD4 T cells (Tn) activated by
antigen presented on immature DCs that do not
produce IL-6 production are induced by TGF-ß to
express Foxp3 and develop into aTregs (top
panel). Tns activated by mature, TLR-activated
DCs that produce IL-6 are induced by TGF-ß to
upregulate IL-23R and become competent for IL-17
production and IL-23 signaling. IL-23 signaling
induces responsiveness to IL-18 and IL-1, which
can act synergistically with IL-23 to induce Th17
cytokine production independently of TCR
stimulation. Alternatively, TCR stimulation by
antigen can induce Th17 cytokine production
directly, without a requirement for IL-23, IL-1,
or IL-18. Dotted lines indicate possible positive
feedback loops by which cytokine products of Th17
(IL-6) or aTreg cells (TGF-ß1) may reinforce
lineage development.
13
Factors that influence T cell lineage commitment
- to maintain self-tolerance or to initiate an
immune response against foreign Ag, T cells must
recognize MHC molecules loaded with self or
non-self - peptides on the surface of APCs. DCs are
professional APCs with the unique ability to
prime naïve T cells. - Immunogenic DCs stimulate and bias naïve CD4 T
cells into TH1/TH2 cells and naïve CD8 T cells
into cytotoxic T cells. - Regulatory DCs trigger apoptosis/anergy of
Ag-specific T cells, and induce/amplify T regs. - During the DCT cell interaction several factors
influence the ability of DC to activate/bias TH
cells - 1. The density/affinity of the MHC-peptide
complex for the TCR - 2. The level of costimulatory molecules
expressed by DCs - 3. The length of DCT cell contact
- 4. The DCT cell ratio
- 5. The cytokines secreted by the DCs and the
neighbouring cells, e.g. secretion of IL-12p70 or
the presence of IL-4 - induces differentiation of TH1 or TH2
cells, respectively release of TGFß is
associated with generation of Tregs.
- in the absence of infection DCs secrete
predominantly high TGF-ß, low IL-6, low IL-23 - Under these conditions CD4 T cells are
activated to express FOXP3 and exhibit a
regulatory phenotype - in early infection DCs express high levels of
IL-6, TGF-ß and IL-23 - Under these conditions naïve CD4 T cells
respond by expressing ROR?t and become TH17 cells
(TNF inhibits TH17) - DIFFERENTIATION OF NAÏVE T CELLS INTO EFFECTOR T
CELLS IS INFLUENCED BY CYTOKINES INVOKED BY
PATHOGENS
14
The JAK-STAT Pathway
JAK
JAK
P
P
JAK
JAK
JAK
JAK
P
P
P
P
P
P
Y
P
P
Y
STAT
STAT
RECEPTOR
P
Y
CYTOPLASM
STAT
STAT
Y
P
NUCLEUS
P
Y
STAT
STAT
Y
P
DNA