Chapter 17' Reactions of Aromatic Compounds - PowerPoint PPT Presentation
1 / 55
Title:
Chapter 17' Reactions of Aromatic Compounds
Description:
b. mechanism - setting up th electrophile (from H2SO4) Chapter 17. 8. 5. Halogenation ... But -Cl, -Br, -I slow down electrophilic substitution ... – PowerPoint PPT presentation
Number of Views:357
Avg rating:3.0/5.0
Title: Chapter 17' Reactions of Aromatic Compounds
1
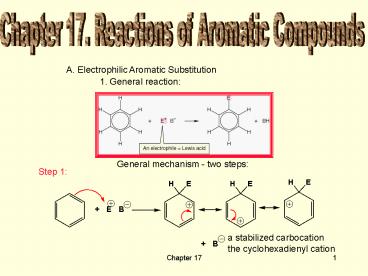
Chapter 17. Reactions of Aromatic Compounds
A. Electrophilic Aromatic Substitution
1. General reaction
General mechanism - two steps
Step 1
a stabilized carbocation the cyclohexadienyl
cation
2
Step 2
In terms of an energy/reaction path diagram
3
2. Reaction with strong acids (H2SO4, HBr, etc)
E H
4
But why not addition reactions????
nonaromatic
This is a common pattern for aromatic compounds -
substitution regenerates aromatic, stabilized
compounds
5
3. Sulfonation a. overall reaction - fuming
sulfuric acid
b. mechanism
6
c. The reverse reaction - we will see why this
reaction is important a little later -
desulfonation
4. A horse of a different color - nitration a.
overall reaction - note use of H2SO4!
7
b. mechanism - setting up th electrophile
(from H2SO4)
8
5. Halogenation
a. overall reaction
b. mechanism
9
c. iodination
6. Friedal - Crafts Alkylation
a. overall reaction
X Cl, Br
10
b. mechanism
11
c. potential problems.
12
7. Friedal - Crafts Acylation
a. overall reaction
13
(a key feature)
b. mechanism
14
c. Gatterman - Koch formylation
formyl chloride
15
B. Directing effects in electrophilic aromatic
substitution reactions For di-substituted
arenes recall that there are three isomers
So, for anisole
The methoxy group directs ortho, para.
16
(No Transcript)
17
There are two ways to look at these patterns. 1.
p-donating substituents - lets use E as the
electrophile
18
ortho substitution
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
(No Transcript)
23
(No Transcript)
24
But -Cl, -Br, -I slow down electrophilic
substitution
This brings up the other way to look at o, p
activating groups
E prefers to attack electron rich carbons
25
2. p-electron withdrawing
E prefers to attack electron rich carbons - so
meta substitution is preferred!
26
The alternative argument goes like this
(same thing happens at ortho attack)
27
(No Transcript)
28
Lets review!!
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
3. Overview
33
C. Polysubstitution of aromatic compounds. A
general rule of thumb 1. Activating o,p
directors are stronger than the meta directing
deactivators. 2. The alkyl groups and
halides are in-between. 3. Steric effects can be
important.
Some simple examples - the directors work
together
34
(No Transcript)
35
(No Transcript)
36
Some not - so - simple examples and tricks!
37
(No Transcript)
38
junk, tar
39
D. Clemenson reduction
1. general reaction
2. synthetic uses strategy
40
(No Transcript)
41
E. Nucleophilic Aromatic Substitution
NOT A GENERAL NOR A GOOD SYNTHETIC METHOD!!!
1. general reaction
BUT
and
42
2. mechanism - there are TWO!!
- Normal path - for activated arenes (ones
- with a number of electron withdrawing groups
- and one leaving group).
43
b. the benzyne mechanism - elimination/ addition
sequence - needs a very, very strong base and a
leaving group.
Mechanism was proven by J. D. Roberts using
13C labels
44
(No Transcript)
45
F. Other addition reactions - reduction 1.
catalytic reduction
46
2. Birch reduction a. overall reaction
b. mechanism
47
(No Transcript)
48
c. Substituted variants p-donors stay at CC p
electron withdrawers get left at reduced
positions
49
G. Reactions at the benzylic position 1.
oxidation
(R can be any alkyl group)
2. halogenation
50
(No Transcript)
51
3. nucleophilic substitution
R- relative rate CH3CH2-
1.0 (CH3)2CH- 0.04 allyl-
33 benzyl 78
(SN2)
52
H. Reactions of phenols 1. acidity
Why??
53
3. oxidants
- Summary
- 1. General features of electrophilic aromatic
substitution - a. mechanism - general
- b. directing effects which are which, why
54
2. Specific reactions a. sulfonation b.
nitration c. halogenation d. Friedal-Crafts
alkylation e. Friedal-Crafts acylation f.
Gatterman-Koch synthesis 3. Synthesis of
poly-substituted aromatic compounds 4. Clemenson
reduction 5. Nucleophilic aromatic
substitution a. addition-elimination
mechanism b. benzyne mechanism 6. Hydrogenation
of arenes a. catalytic hydrogenation b. Birch
reduction
55
7. Benzylic reactions a. oxidation b.
halogenation c. nucleophilic substitution 8.
Reactions of phenols a. use as a nucleophile b.
formation of quinones