Immunoprecipitation Workflow and Troubleshooting Tips - PowerPoint PPT Presentation
Title:
Immunoprecipitation Workflow and Troubleshooting Tips
Description:
Immunoprecipitation Workflow and Troubleshooting Tips – PowerPoint PPT presentation
Number of Views:4
Title: Immunoprecipitation Workflow and Troubleshooting Tips
1
Immunoprecipitation Workflow and Troubleshooting
Tips
CD
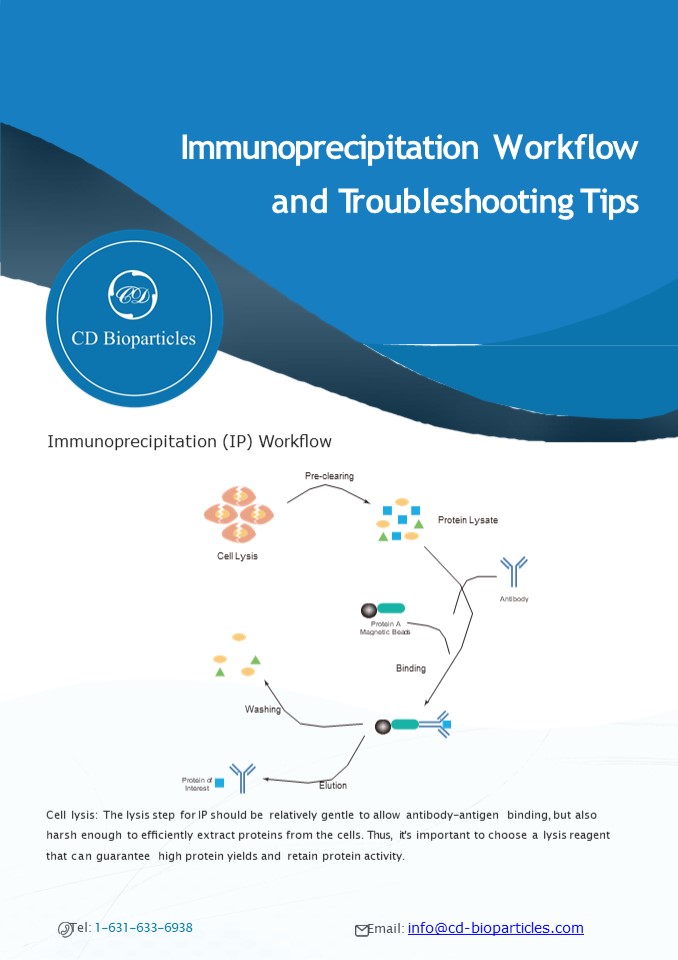
Immunoprecipitation (IP) Work?ow Pre-clearing
Protein Lysate
Cell Lysis
Antibody
Protein A Magnetic Beads
Binding
Washing
Protein of Interest
Elution
Cell lysis The lysis step for IP should be
relatively gentle to allow antibody-antigen
binding, but also harsh enough to ef?ciently
extract proteins from the cells. Thus, it's
important to choose a lysis reagent that can
guarantee high protein yields and retain protein
activity.
Email info_at_cd-bioparticles.com
2
- Pre-clearing This step reduces the number of
non-speci?c contaminants in the cell lysate as
well as removes proteins that possess a higher
af?nity to protein G or protein A before the
speci?c IP steps. As a result of pre-cleaning,
there would be less background noise and an
improved signal-to-noise ratio. - Binding Magnetic beads (or agarose), antibody,
and antigen form complexes at this step. The
buffers used here and at the washing steps are
vital to perform a successful IP. The order that
these three components are added also affect the
results. Antibodies may be added ?rst to
covalently or noncovalently bind to the magnetic
beads before adding lysate, or incubated with
lysate to form a complex before adding protein A
or protein G magnetic beads to purify the complex
from the mixture. - Washing Ideally, this step can break all
nonspeci?c bindings without affecting the speci?c
interactions between antibody and antigen.
Adding extra lysis buffer to the washing buffer
can help breaking the nonspeci?c bindings. - Elution Generally, there are three kinds of
elution buffers, including glycine, SDS, and urea
elution. Any of the three elution buffers can be
used to elute the protein from the beads. The SDS
buffer is harsh enough to elute noncovalently
bound antibodies, antibody fragments, and the
protein of interest. The glycine buffer is a
gentler choice. - Troubleshooting Tips
- 1. To improve elution conditions
- Choose an appropriate lysis buffer according to
the protein location (membrane, cytosolic, or
nucleus). The pH, detergent, salt, and divalent
cation concentrations should be optimized for
each IP. - Check the antibody binding properties of each
beads. Its important to match the binding
speci?city of the beads to the species and the
antibody subtypes (Table 1).
Email info_at_cd-bioparticles.com
3
Table 1. Immunoglobulin binding
properties. Protein A Protein G
- - - - - - - -
- - - -
- -
Species Immunoglobulin Isotype
Protein A/G
Human IgG1
Human IgG2
Human IgG3
Human IgG4
Human IgM
Human IgE
Human IgD
-
Human IgA
Human IgA1
Human IgA2
Human Fab
Human ScFv
Mouse IgG1
Mouse IgG2a
Mouse IgG2b
Mouse IgG3
Mouse IgM
-
Rat IgG1
Rat IgG2a
Rat IgG2b
Rat IgG2c
Cow IgG1
Cow IgG2
Sheep IgG1
Sheep IgG2
Goat IgG1
Goat IgG2
Chicken IgY
-
Hamster IgG
Pig IgG
Horse IgG
Rabbit IgG
Cat IgG
Rhesus Monkey IgG
Strong binding Medium binding Weak
binding - No binding.
Email info_at_cd-bioparticles.com
4
- Alter the components, salt concentration, or pH
of the elution buffer if no protein of interest
is eluted from the beads. - Conduct a titration experiment ?rst to optimize
the antibody concentration if there isnt enough
antibodies for proper binding. - To enhance the expression of protein of interest,
increase the volume of the cell lysate and
pre-clearing the sample to decrease non-speci?c
binding and remove debris. - Polyclonal antibodies usually perform better than
monoclonal antibodies. - Spin lysate for 30 min before adding the
antibody. This can remove insoluble proteins,
membrane fragments, and debris to reduce the
number of the competing proteins in the sample. - Primary antibody and antigen of interest can be
incubated from 4 hours to overnight at 4ºC. - Avoid using lysates containing substances such as
dithiothreitol, 2-mercaptoethanol, or other reduc
ing agents. This will affect antibody binding.
Extremes in pH and excessive detergent
concentrations also affect antibody-antigen
interaction. - 2. High background
- Remove supernatant immediately after
centrifugations to avoid carryover of
detergent-insoluble proteins. - To thoroughly wash the samples, place a lid on
the tube and invert several times before
centrifugation. - Pre-blocking beads with fresh BSA can decrease
non-speci?c protein binding to the beads. Beads
are incubated with 1 BSA in PBS for an hour and
then wash 3 to 4 times in PBS before use. - Use an af?nity-puri?ed antibody with high
speci?city to avoid high background. - Use recommended numbers of antibody or optimize
the concentration of the antibody by titration.
Using too much antibody may cause non-speci?c
binding. - Decrease cell numbers to avoid high protein
concentration in the lysate. - In case of too much non-speci?c binding of
proteins to antibody, use an irrelevant antibody
of the same species of origin and the same Ig
subclass to pre-clear the lysate. - Add fresh protease inhibitors in the sample
lysate to prevent antigen degrading during IP. - Inappropriate washing may cause high background.
Use distilled water and increase the number of
washes. Before the last wash, it is a good
practice to transfer the cell pellet to a new
tube.
Email info_at_cd-bioparticles.com
5
4. Antibody heavy/light chains background If the
target protein is about 25kDa or 50kDa, the
detection signal will be masked by the antibody
chain. There are two solutions to this problem
1) use capture and detection antibody originated
from different species 2) crosslink the capture
antibody with protein beads and use these
conjugates to precipitate the protein of
interest, if using antibodies from the same
species. Creative Diagnostics provides a
comprehensive list of af?nity magnetic particles
for immunoprecipitation and protein separation,
including protein A and protein G magnetic
particles in wide range of sizes. Please visit
our website to see more.
For more information, view our website
www.cd-bioparticles.com
Email info_at_cd-bioparticles.com
Tel 1-631-633-6938 Fax 1-631-938-8221
Address 45-1 Ramsey Road, Shirley, NY 11967, USA
5