What is a Lithium Ion Battery?
Title:
What is a Lithium Ion Battery?
Description:
The lithium-ion battery was created in 1991 by Sony Corp. This type of battery has become ubiquitous throughout our lives because they’re efficient and long-lasting. However, these batteries have downsides. For example, they can overheat when charging or discharging at high speeds. Also, they can catch fire if damaged. –
Number of Views:15
Title: What is a Lithium Ion Battery?
1
What is a Lithium Ion Battery?
2
However, these batteries have downsides. For
example, they can overheat when charging or
discharging at high speeds. Also, they can catch
fire if damaged. Lithium batteries are
rechargeable batteries that have become
increasingly popular over the past few years.
These batteries offer many advantages, including
extended battery life, high capacity, low
self-discharge rate, and fast charging time.
However, they do not last forever and must be
replaced after a certain amount of use. If you
want to know what lithium-ion batteries are and
how they work, keep reading!
3
Types of Batteries Three types of lithium-ion
batteries exist cylindrical, prismatic, and
pouch. Each type offers its advantages and
disadvantages. Cylindrical batteries are commonly
used in cell phones and laptops. They are
generally smaller than prismatic and pouch cells
and have a higher capacity per unit volume.
Prismatic batteries are often used in cameras and
camcorders. Pouch cells are best suited for
portable devices because they are small,
lightweight, and durable.
4
Advantages of Lithium Ion Batteries Lithium-ion
cells have some significant advantages. They are
inexpensive, safe, and environmentally friendly.
They are also highly reliable and have a long
lifespan. In addition, they are capable of being
recharged thousands of times.
5
Disadvantages of Lithium Ion Cells Battery
technology consulting states that despite their
many advantages, lithium-ion cells have some
drawbacks. They are expensive and require special
care. They also have a short shelf life and
cannot be stored at extreme temperatures.
6
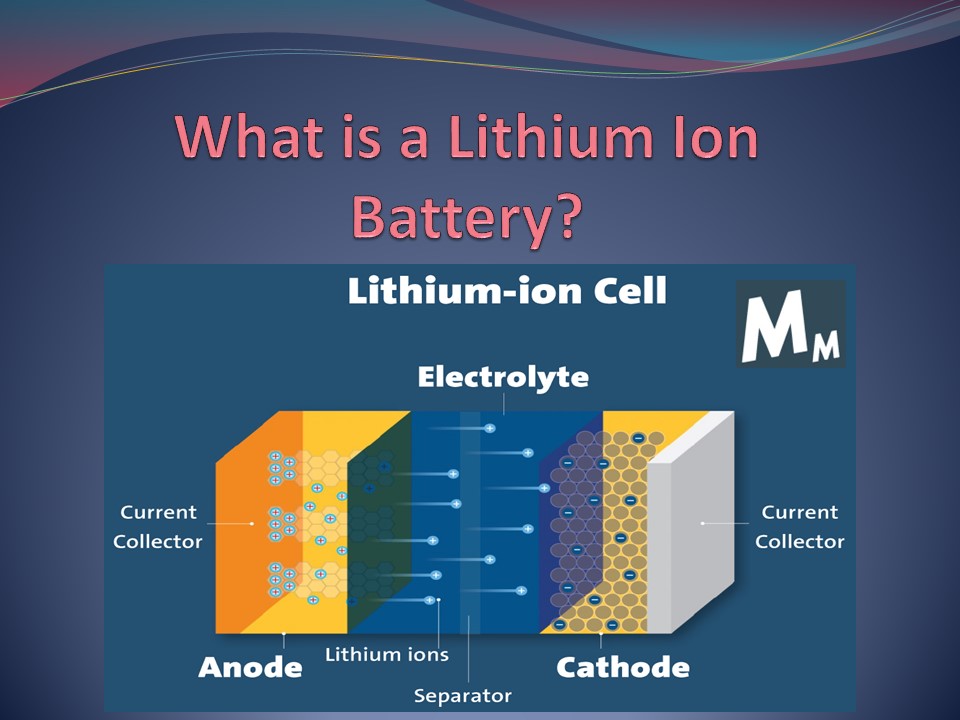
How Do They Work?
When current flows through a lithium-ion battery,
electrons flow from the negative electrode
(anode) to the positive electrode (cathode). As
these electrons pass through the separator, they
create a chemical reaction called oxidation and
reduction. Oxidation occurs when electrons
combine with oxygen atoms to form molecules of
water. Reduction occurs when electrons combine
with hydrogen atoms to form hydrogen gas
molecules. Lithium batteries are a high-energy,
high-density rechargeable battery that uses
lithium ions as its vital component. During a
discharge cycle, lithium atoms in the anode are
ionized and separated from their electrons. The
lithium ions move from the anode and pass through
the electrolyte until they reach the cathode,
recombining with their electrons and electrically
neutralising. Because of lithiums small size
(third only to hydrogen and helium), Li-ion
batteries can have a very high voltage and charge
storage per unit mass and volume.
7
Thank You For Watching