January,07
1 / 40
Title: January,07
1
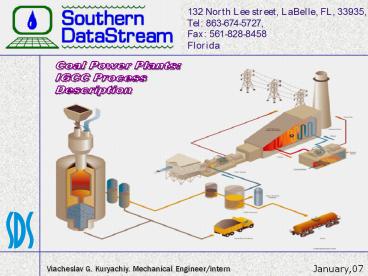
Coal Power Plants IGCC Process Description
Viacheslav G. Kuryachiy. Mechanical
Engineer/intern
January,07
2
2
Common structure of IGCC
3
IGCC POWER PLANT UNITS
1. Air separation Unit (ASU) 2.
Gasification Unit 3. Gas Cooling unit 4.
Gas Cleaning Unit 5. Sulfur Removing
Unit 6. Gas Combustion Turbine Unit 7.
Heat Recovery Steam Generator Unit 8. Steam
Turbine Unit
3
4
Possible variants of Integrated Gasification
Combine Cycle at Power Plants
Source 2003 Electric Power Research Institute,
Inc. http//www.iea.org/dbtw-wpd/Textbase/work/200
4/zets/technical/holt.pdf
4
5
1. Air separation Unit (ASU)
The oxygen plant consists of a conventional,
cryogenic air separation unit (ASU). The primary
purpose of the ASU is to produce pressurized
oxygen gas for the reaction with the coal slurry
in the Gasifier and to produce nitrogen gas that
is used as a diluent in the combustion turbine to
control peak flame temperature thereby reducing
the formation of NO, during syngas combustion.
Secondary uses include low pressure oxygen for
optimizing the Sulfur Recovery system and low
pressure nitrogen for purging operations.
Dulient Nitrogen to combuster
Oxygen to gasifier
Source http//valleywatch.net/valleywatch/docs/Du
ke20Edw.20Application/Chapter2.pdf
5
6
Air separation Unit (ASU)
The design for the air separation unit (ASU) is
based on a high-pressure ASU with half of the air
required by the ASU supplied from the combustion
turbine. This level of integration was chosen to
allow the ASU to operate independently of the gas
turbine, but still obtain the efficiency
advantage of an integrated system.
Source ECOS 2000, University of Twente Enschede,
The Netherlands, July 5-7, 2000 http//www.airprod
ucts.com/NR/rdonlyres/6753032F-A8B8-4339-A12F-BB1A
8DB46735/0/ECOS.pdf
6
7
2. Gasification Unit
Coal Slurry
O2 from ASU
Coal, water and oxygen are fed into a
high-pressure gasifier, where the coal is
partially combusted and converted into synthetic
gas (syngas). The ash in the coal is converted
to inert, glassy slag.
Feed Water
Radiant Syngas Cooler
- 2.1. Classification of gasifiers.
- Fixed bed gasifiers
- Fluidized bed gasifiers
- Entrained flow (slagging) gasifiers.
High Pressure Steam
Syngas
Slag
Source The U.S. Department of Energy and Tampa
Electric Company http//www.fossil.energy.gov/prog
rams/powersystems/publications/Clean_Coal_Topical_
Reports/topical19.pdf
7
8
Gasification Unit
Moving-Bad Gasifier (Dry Ash)
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
8
9
Gasification Unit
Fluidized-Bad Gasifier
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
9
10
Gasification Unit
Entrained-Bed Gasifier
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
10
11
Gasification Unit
At the outlet of the gasifier reactor the
temperature of the syngas is around 1500 C and
the fly ash (or slag) is in liquid form. To
protect downstream process equipment from
fouling, a quench is needed to solidify the slag
and make it non-sticky. There are four main
alternatives for quenching - Radiant syngas
cooling - Water quench - Gas recycle quench
- Chemical quench
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
11
12
Gasification Unit
Unless the hot syngas has been totally quenched
with water, it typically has a temperature of
around 900 C and therefore needs further cooling
before downstream gas clean up processes. There
are two classes of syngas coolers for steam
production - Fire tube boilers - Water
tube boilers Both types have been operated
successfully in different plants. Of the two
types, fire tube boilers are lower in cost. In
this design, the hot raw syngas flows inside the
tubes, while high pressure steam is generated on
the outside. This means that the tubes are
subjected to an external pressure. Depending on
the design, maximum steam pressure is between 100
and 150 bar. Water tube boilers can handle higher
steam pressure.
12
13
Gasification Unit
In the table below represented gasifiers from
three biggest companies
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
13
14
Gasification Unit
TYPES OF COAL
High Ranked Coal - Bituminous and Anthracite
coal (Eastern Coal) - About 50 of the U.S.
coal reserves ? Higher heating value ? Lower
moisture content ? Lower mineral content ?
Performs well in slagging gasifiers Low Ranked
Coal - Lignite and Sub-bituminous coal
(Western Coal) - About 50 of the U.S. coal
reserves - Does not perform well in slagging
gasifiers ? Sasol fixed bed ? Transport
gasifier (under development)
Source http//www.netl.doe.gov/technologies/coalp
ower/gasification/pubs/pdf/06051620FSO20Presenta
tion.pdf
14
15
3. Gas Cooling Unit
Gas cooling unit consists of low temperature gas
cooling (LTGC), COS hydrolysis and Mercury
removal.
3.1. LTGC In this section, the particulate-free
syngas from the syngas scrubber is cooled and the
heat is recovered by heating clean syngas and
generating steam. Most of the water in the syngas
is condensed and removed before it reaches the
Acid Gas Removal (AGR) section.
Source http//valleywatch.net/valleywatch/docs/Du
ke20Edw.20Application/Chapter2.pdf
15
16
Gas Cooling Unit
3.2. COS Hydrolysis Each train of gas cooling
contains a reactor for conversion of Carbonyl
Sulfide (COS) to Hydrogen Sulfide (H2S) to enable
more complete sulfur removal in the AGR.
Source http//valleywatch.net/valleywatch/docs/Du
ke20Edw.20Application/Chapter2.pdf
16
17
Gas Cooling Unit
3.3. Mercury removal
Carbon beds remove 90-95 of the mercury from
coal derived synthesis gas. Mercury sulfide on
the spent carbon is stable and currently the best
option is to dispose of it at certified storage
sites. Regeneration with mercury recovery is
complex and expensive.
The carbon is impregnated with sulfur at a
concentration of about 10-15 wt. The mercury
reacts with sulfur as the gas goes through the
sulfur bed to form HgS. After the sulfur on the
carbon is exhausted, the spent adsorbent is
shipped to a hazardous chemicals disposal
site. HgS is a very stable compound and its
long-term storage presents no problems. The spent
carbon can also be incinerated and the mercury
recovered from the incinerator gas via cooling
and condensation.
Source http//valleywatch.net/valleywatch/docs/Du
ke20Edw.20Application/Chapter2.pdf
17
18
Gas Cooling Unit
Examples of syngas composition at scrubber outlet
(mole )
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
18
19
4. Gas Cleaning Unit
The syngas is cleaned of particles. Next the
syngas passes through a bed of activated
charcoal, which captures the mercury. The sulfur
is removed from the syngas and converted to
either elemental sulfur or sulfuric acid for sale
to chemical companies or fertilizer companies.
- Particle removal
- Metal Carbonyls
- HCl Removal
- Shift Rx option
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
19
20
Gas Cleaning Unit
- Particle removal
Dry solids removal systems use candle filters
that can remove all solids from the gas at
temperatures between 300 and 500 C. Above 500
C, alkali compounds may pass the filters in
significant amounts. Below 300 C, the filters
may be blinded of deposits of ammonium chloride
(NH4Cl). Including cyclones upstream will reduce
the loading on the filters and therefore also the
risk of breakage. Wet solids removal systems use
water scrubbers operating at a temperature lower
than the dew point of the gas so that the
smallest solid particles can act as nuclei for
condensation and ensure efficient operation.
Even if an IGCC plant has a candle filter it
usually also adds a wet scrubbing system for
removal of remaining impurities such as chlorides
and ammonia.
20
21
Gas Cleaning Unit
- Metal Carbonyls
Iron and nickel carbonyls (Fe(CO)5, Ni(CO)4) are
both undesirable trace components in synthesis
gases. Metal carbonyls are often present in
synthesis gas that is made from petroleum
residues. Nickel carbonyls are damaging to
combustion turbines but can be removed from
synthesis gas by activated carbon.
Metal carbonyls can also be absorbed by
low-temperature solvents such as used by the
Rectisol process. These metal compounds, if not
removed, wind up in the Claus plant feed, and
are burned to FeS and NiS and then deposited on
the Claus catalyst. Both of these outcomes are
undesirable.
21
22
Gas Cleaning Unit
- HCL removal
Nahcolite (naturally occurring sodium
bicarbonate), Trona (naturally occurring sodium
sesquicarbonate), synthetic sodium
carbonate/bicarbonate mixtures, Ca(OH)2, and
other sorbents are effective for dry removal of
HCl and HF from syngas. Sorbent requirements and
performance depends on the gas conditions and
the contaminant concentrations. Injection before
the filter is necessary.
22
23
Gas Cleaning Unit
- Shift Rx option
Shift Rx the additional process block for CO2
capture. It is a shift reactor in which the CO
reacts with H2O to H2 and CO2. In the shift
reactor, the heating value of the CO is
transferred to H2 and the carbon atoms end up in
the CO2 molecules. It has been found that a so
called sour shift upstream the sulfur removal is
more energy efficient and has lower cost than a
clean shift downstream of the sulfur removal.
COH2O? H2CO2 - 41.2 MJ/kmol (exothermic
reaction )
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
23
24
Gas Cleaning Unit
- Shift Rx option IGCC with CO2 capture
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
24
25
5. Sulfur Removal Unit
The sulfur is removed from the syngas and
converted to either elemental sulfur or sulfuric
acid for sale to chemical companies or fertilizer
companies.
- Sulfur removal
- Acid gas removal (AGR)
- Sulfur recovery unit (SRU)
- Tail gas treating (TGT)
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
25
26
Sulfur Removal Unit
Acid Gas Removal (AGR) Currently, the processes
of choice in commercial IGCC facilities for the
removal of acid gases are both the chemical
solvent AGR processes based on aqueous
methyldiethanolamine (MDEA) and the physical
solvent-based Selexol processwhich uses mixtures
of dimethyl ethers of polyethylene glycol. In
most of the IGCC applications now, with both of
these AGR processes, the AGR units are preceded
by carbonyl sulfide (COS) hydrolysis units to
convert most of the COS to H2S. This then enables
the AGR units to accomplish deeper total sulfur
removal and lower H2S levels. Total sulfur
(COSH2S) levels of lt20 ppmv may be required if
selective catalytic reduction (SCR) is to be used
with IGCCto prevent ammonium sulfate salt
deposition and corrosion problems in the colder
sections of the heat recovery steam generator
(HRSG).
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
26
27
Gas Cleaning Unit
- Sulfur removal
The AGR process removes the sulfur from the
syngas. In current GCC plants, the two
processes of choice are based on absorption in
a liquid solvent - Chemical solvents
based on aqueous methyldiethanolamine (MDEA)
- The Selexol process based on a physical
solvent - Rectisol - Mixed
chemical/physical. The first both methods are
capable of reducing total sulfur (H2S COS) to
levels below 20 ppmv in the cleaned syngas. For
CO2 capture a second stage AGR would be added to
remove the CO2 from the sulfur free syngas. If
the syngas will be used to produce chemicals,
deep sulfur removal will be required to protect
the catalyst downstream. In this case the more
expensive Rectisol physical solvent AGR process
may be applied. This process is a standard
solution in chemical applications such as
methanol and ammonia. Chemical solvents AGR
processes also require steam in the stripping
process to regenerate the solvent, while physical
solvents are regenerated only by staged flashing
techniques.
Source http//www.netl.doe.gov/technologies/coalp
ower/gasification/pubs/pdf/
SFA20Pacific_Process20Screening20Analysis_Dec2
02002.pdf
27
28
Gas Cleaning Unit
- Sulfur removal
The AGR process. In CO2 removal applications,
the Selexol process is chilled thus
facilitating deep H2S removal as well as CO2
removal. The Rectisol physical solvent AGR
process based on low-temperature (refrigerated)
methanol is capable of deep total sulfur
removal, but it is regarded as the most expensive
AGR process. Therefore, its use is generally
reserved for chemical synthesis gas applications
in which very pure syngas is required. Its use in
IGCCs with CO2 removal has also been proposed.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
28
29
Gas Cleaning Unit
- Sulfur removal
- Methyldiethanolamine (MDEA)
MDEA is the amine scrubbing process. It does not
combine with COS chemically. Only limited
physical COS absorption takes place with MDEA.
COS can be physically removed by MDEA only with
very high solvent circulation rates, at which
point the CO2 is also removed quantitatively. For
synthesis gases that contain appreciable
quantities of COS, prior removal of the COS is
usually required. A catalytic hydrolysis unit is
usually employed ahead of the MDEA unit as was
done at the Tampa Electric gasification plants.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
29
30
Gas Cleaning Unit
- Sulfur removal
- Selexol process The Selexol
process solvent is a mixture of dimethyl ethers
of polyethylene glycol, and has the formulation
CH3O(CH2CH2O)nCH3 where n is between 3 and
9. The Selexol process uses a physical solvent
to remove acid gas from streams of synthetic or
natural gas. The process may be regenerated
either thermally, by flashing, or by stripping
gas. The Selexol process is ideally suited for
the selective removal of H2S and other sulfur
compounds, or for the bulk removal of CO2.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
30
31
Gas Cleaning Unit
- Sulfur removal
- Selexol process Acid gas
partial pressure is the key driving force for the
Selexol process. Typical feed conditions range
between 300 and 2000 psia with acid gas
composition (CO2 H2S) from 5 to more than 60
by volume. The product specifications achievable
depend on the application and can be anywhere
from ppmv up to percent levels of acid gas.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
31
32
Gas Cleaning Unit
- Sulfur removal
- Selexol process Acid gas
partial pressure is the key driving force for the
Selexol process. Typical feed conditions range
between 300 and 2000 psia with acid gas
composition (CO2 H2S) from 5 to more than 60
by volume. The product specifications achievable
depend on the application and can be anywhere
from ppmv up to percent levels of acid gas.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
32
33
Gas Cleaning Unit
- Sulfur removal
- Recticol process The
Rectisol process uses chilled methanol at a
temperature of about -40F to -80F. Methanols
selectivity for H2S over CO2 at these
temperatures is about 6/1, a little lower than
that of Selexol at its usual operating
temperature. However, the solubilities of H2S and
COS in methanol, at typical process operating
temperatures, are higher than in Selexol and
allow for very deep sulfur removal (lt0.1 ppmv H2S
plus COS). Rectisols high selectivity for H2S
over CO2, combined with the ability to remove
COS, is the primary advantage of the process.
Chilled methanol also absorbs HCN, NH3, and iron-
and nickel-carbonyls. The solubilities of these
trace components and other organic sulfur
compounds are even higher than that of H2S.
Rectisols complex scheme and the need to
refrigerate the solvent are its main
disadvantages, resulting in high capital and
operating costs.
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
33
34
Gas Cleaning Unit
- Sulfur removal
- Mixed chemical/physical The
mixed chemical/physical processes usually employ
mixtures of an amine and a physical solvent in an
effort to capture the best characteristics of
each solvent. The best known example of the
mixed/chemical solvent process is Sulfinol, a
mixture of sulfolane (tetrahydrothiophene
dioxide) and an aqueous solution of an amine,
either DIPA (diisopropanol amine) or MDEA.
34
35
Gas Cleaning Unit
- Sulfur recovery unit (SRU)
The standard solution for the SRU is the Claus
process which produces elemental sulfur from H2S
by substoichiometric combustion with air or
oxygen. Many versions of this process are
available. The sulfur may be fixed as elemental
sulfur in liquid or solid form, or as sulfuric
acid. The thermodynamics of the Claus process is
such that one does not achieve high enough
degrees of sulfur recovery without some treating
of the tail gas, which usually contain mostly H2S
and SO2, but also small amounts of COS, CS2 and
elemental sulfur vapors.
35
36
Gas Cleaning Unit
- Tail gas treating (TGT)
The principal current tail gas treating approach
is to hydrogenate/hydrolyze the sulfur species in
the tail gas and then scrub out the resulting H2S
in an acid gas removal absorber. This approach is
capable of boosting total sulfur recovery to well
over 99.9. In some IGCC plant designs Claus tail
gas, after H2S scrubbing, is compressed and
routed to the combustion turbine. This tail gas
handling eliminates the need for a tail gas
incinerator and provides additional fuel and mass
flow to the combustion turbine. H2S can be
scrubbed in a stand alone separate process, or
could be routed to the acid gas removal unit
upstream of the Claus plant. Tail gas treating of
this type will remain the dominant and a required
step in order to meet the proposed regulations.
36
37
Gas Cleaning Unit
- Tail gas treating (TGT)
TGT alternatives a) Dedicated absorber for H2S
in TGT. b) Integration with
upstream absorber for H2S capture
Source Massachusetts Institute of Technology.
Laboratory for Energy and the Environment. http//
lfee.mit.edu/publications Publication No. LFEE
2005-002 WP http//lfee.mit.edu/public/LFEE_2005-0
02_WP5.pdf
37
38
6. Gas Combustion Turbine Unit
The gas turbine is integrated with the Air
Separation Unit.
From the
compressor exhaust, a bleed stream is used to
supply 50 of the air supply needed for the
ASU. An additional bleed, 14 of the compressor
discharge air, is chilled to 600 F and used for
cooling in the turbine expander. Heat recovered
from the air cooler is used in the steam cycle.
The compressor discharge supplies air for use in
the HGCU regenerator. The remainder of the
compressor discharge air is used to combust the
clean fuel gas. The ASU returns a nitrogen stream
to the gas turbine combustor to assist in NOX
control and to increase the flow rate and the
power generated in the turbine expander.
Combustor duct cooling is accomplished using
intermediate pressure steam supplied from the
steam bottoming cycle. This reheated steam is
returned to the steam cycle. The combustor
exhaust gases enter the expander where energy is
recovered to produce power.
38
39
7. Heat Recovery Steam Generator
The gas turbine exhausts into a HRSG (Heat
Recovery Steam Generator), which generates high
temperature superheated steam. This steam is
injected into the gas turbine and expanded
through it to increase the electrical power
output. Variations in process steam requirements
are handled by varying the fuel input to the duct
burner located between the superheater and the
evaporator. A simple cogeneration system
typically consists of a HRSG located behind a gas
turbine. This system is efficient when the
thermal load is held nearly constant. When the
thermal load drops below the design point the
operating economics of the system are penalized.
Cheng Cycle System
Source The American Society of Mechanical
Engineers. Industrial Power Conference - PWRVol.
4 Editor B. L. http//www.ecoxy.com/download/heat
_recovery_steam_generator_for_cheng.pdf
39
40
8. Steam Turbine Unit
The steam turbine converts heat energy into
mechanical motion.
Steam turbine is an action turbine (no reaction
turbine), i.e. the steam jet meets from a being
certain nozzle the freely turning impeller.
There's a high pressure in front of the turbine,
while behind it a low pressure is maintained, so
there's a pressure gradient Steam shoots through
the turbine to the rear end. It delivers kinetic
energy to the impeller and cools down thereby.
Steam is produced in Gas Combustion Turbine. Then
it heated in HRSG unit and goes to the steam
turbine. The mechanical energy from the turbine
through the generator converts to electrical
energy. Steam doesn't escape then, it is
condensed in a condensor and then pushed back
into the cycle.
40