Conductivity of Solutions - PowerPoint PPT Presentation
1 / 24
Title:
Conductivity of Solutions
Description:
CHAPTER 14. Ions in Aqueous Solutions and Colligative Properties ... Butyric acid rancid butter. Amino acids protein. Nucleic acids DNA and RNA ... – PowerPoint PPT presentation
Number of Views:461
Avg rating:3.0/5.0
Title: Conductivity of Solutions
1
CHAPTER 14
Ions in Aqueous Solutions and Colligative
Properties
2
Dissociation Equations
NaCl(s) ?
Na(aq) Cl-(aq)
AgNO3(s) ?
Ag(aq) NO3-(aq)
MgCl2(s) ?
Mg2(aq) 2 Cl-(aq)
Na2SO4(s) ?
2 Na(aq) SO42-(aq)
AlCl3(s) ?
Al3(aq) 3 Cl-(aq)
3
Dissolution of sodium Chloride
4
Double replacement forming a precipitate
Double replacement (ionic) equation
Pb(NO3)2(aq) 2KI(aq) ? PbI2(s) 2KNO3(aq)
Complete ionic equation shows compounds as
aqueous ions
Pb2(aq) 2 NO3-(aq) 2 K(aq) 2 I-(aq) ?
PbI2(s) 2K(aq) 2 NO3-(aq)
Net ionic equation eliminates the spectator ions
Pb2(aq) 2 I-(aq) ? PbI2(s)
5
Solubility of Ionic Compounds
6
The ability of a solution to conduct an electric
current can be measured with a simple device.
The ammeter measures the flow of electrons
(current) through the circuit.
- If the ammeter measures a current, and the bulb
- glows, then the solution conducts.
- If the ammeter fails to measure a current, and
the - bulb does not glow, the solution is
non-conducting.
7
Definition of Electrolytes and Nonelectrolytes
An electrolyte is
- A substance whose aqueous solution conducts
- an electric current.
A nonelectrolyte is
- A substance whose aqueous solution does not
- conduct an electric current.
Try to classify the following substances as
electrolytes or nonelectrolytes
8
Electrolytes?
- Pure water
- Tap water
- Sugar solution
- Sodium chloride solution
- Hydrochloric acid solution
- Lactic acid solution
- Ethyl alcohol solution
- Pure sodium chloride
9
Answers to Electrolytes
ELECTROLYTES
NONELECTROLYTES
- Tap water (weak)
- NaCl solution
- HCl solution
- Lactate solution (weak)
- Pure water
- Sugar solution
- Ethanol solution
- Pure NaCl
But why do some compounds conduct electricity
in solution while others do not?
10
Ions tend to stay in solution where they
canconduct a current rather than re-forming a
solid.
The reason for this is the polar nature of the
water molecule
- Positive ions associate with the negative
- end of the water dipole (oxygen).
- Negative ions associate with the positive
- end of the water dipole (hydrogen).
11
Some covalent compounds IONIZE in solution
Covalent acids form ions in solution, with the
help of the water molecules.
For instance, hydrogen chloride molecules, which
are polar, give up their hydrogens to water,
forming chloride ions (Cl-) and hydronium ions
(H3O).
12
Ionization of HCl makes it a strong electrolyte
13
Strong acids such as HCl are completelyionized
in solution.
Other examples of strong acids include
- Sulfuric acid, H2SO4
- Nitric acid, HNO3
- Hydriodic acid, HI
- Perchloric acid, HClO4
14
Weak acids such as lactic acid usually ionize
less than 5 of the time.
Many of these weaker acids are organic
acids that contain a carboxyl group.
The carboxyl group does not easily give up
its hydrogen.
15
Because of the carboxyl group, organic acids
aresometimes called carboxylic acids.
Other organic acids and their sources include
- Citric acid citrus fruit
- Malic acid apples
- Butyric acid rancid butter
- Amino acids protein
- Nucleic acids DNA and RNA
- Ascorbic acid Vitamin C
This is an enormous group of compounds, these
are only a few examples.
16
However, most covalent compounds do not
ionizeat all in solution.
Sugar (sucrose C12H22O11),
and ethanol (ethyl alcohol C2H5OH) do
not ionize -
That is why they are nonelectrolytes!
17
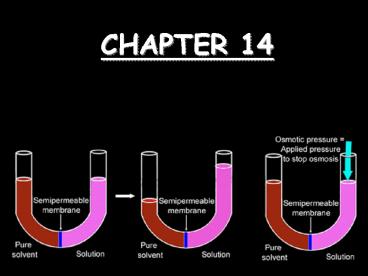
Colligative Properties
Colligative properties are those that depend on
the concentration of particles in a solution, not
upon the identity of those properties.
- Boiling Point Elevation
- Freezing Point Depression
- Osmotic Pressure
18
Freezing Point Depression
Each mole of solute particles lowers the freezing
point of 1 kilogram of water by 1.86 degrees
Celsius.
Kf 1.86 ?C ? kilogram/mol
19
Boiling Point Elevation
Each mole of solute particles raises the boiling
point of 1 kilogram of water by 0.51 degrees
Celsius.
Kb 0.51 ?C ? kilogram/mol
20
Freezing Point Depression and Boiling Point
Elevation Constants
21
The vant Hoff Factor, i
Electrolytes may have two, three or more times
the effect on boiling point and freezing point,
depending on its dissociation.
?T i ? K ? m
22
Dissociation Equations
i 2
NaCl(s) ?
Na(aq) Cl-(aq)
i 2
AgNO3(s) ?
Ag(aq) NO3-(aq)
i 3
MgCl2(s) ?
Mg2(aq) 2 Cl-(aq)
i 3
Na2SO4(s) ?
2 Na(aq) SO42-(aq)
AlCl3(s) ?
Al3(aq) 3 Cl-(aq)
i 4
23
Preventing icing of roads using CaCl2
24
Ideal vs. Real vant Hoff Factor
The ideal vant Hoff Factor is only achieved in
VERY DILUTE solution.