Linear Polarization Resistance LPR and the SternGeary Equation - PowerPoint PPT Presentation
1 / 48
Title:
Linear Polarization Resistance LPR and the SternGeary Equation
Description:
... system can he determined empirically (calibrated from separate weight loss ... The program then fits the best frequency response of the given EIS spectrum, to ... – PowerPoint PPT presentation
Number of Views:1314
Avg rating:3.0/5.0
Title: Linear Polarization Resistance LPR and the SternGeary Equation
1
(No Transcript)
2
(No Transcript)
3
(No Transcript)
4
(No Transcript)
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
(No Transcript)
19
(No Transcript)
20
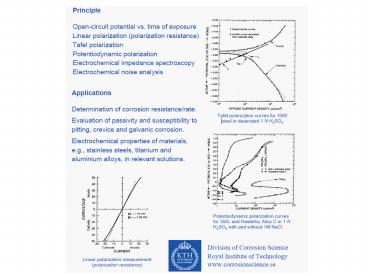
- Linear Polarization Resistance (LPR) and the
Stern-Geary Equation - With this widely used technique in corrosion
monitoring, the polarization resistance of a
material is defined as the slope of the
potential-current density (DE/Di) curve at the
free corrosion potential, yielding the
polarization resistance Rp that can be related
(for reactions under activation control) to the
corrosion current by the Stern-Geary equation - where
- Rp is the polarization resistance
- icorr the corrosion current
- The proportionality constant , B, for a
particular system can he determined empirically
(calibrated from separate weight loss
measurements) or, as shown by Stern and Geary,
can be calculated from ba and bc, the slopes of
the anodic and cathodic Tafel slopes, i.e.
21
- Corrosion Monitoring
- Linear Polarization Resistance (LPR)
- Polarization resistance is particularly useful as
a method to rapidly identify corrosion upsets and
initiate remedial action, thereby prolonging
plant life and minimizing unscheduled downtime.
The technique is utilized to maximum effect, when
installed as a continuous monitoring system. This
technique has been used successfully for over
thirty years, in almost all types of water-based,
corrosive environments. Some of the more common
applications are - Cooling water systems
- Secondary recovery system
- Potable water treatment and distribution systems
- Amine sweetening
- Waste water treatment systems
- Pickling and mineral extraction processes
- Pulp and paper manufacturing
- Hydrocarbon production with free water
- The measurement of polarization resistance has
very similar requirements to the measurement of
full polarization curves. There are essentially
four different methods of making the measurement
according to whether the current or the potential
is controlled and whether the current (or
potential) is swept smoothly from one value to
another, or simply switched between two values.
In addition the measurement may be made between
two nominally identical electrodes (a
two-electrode system), or a conventional
three-electrode system (working, reference and
counter) may be used
22
Electrochemical impedance spectroscopy (EIS) EIS
has been successfully applied to the study of
corrosion systems for thirty years and been
proven to be a powerful and accurate method for
measuring corrosion rates. But in order to access
the charge transfer resistance or polarization
resistance that is proportional to the corrosion
rate at the monitored interface, EIS results have
to be interpreted with the help of a model of the
interface. An important advantage of EIS over
other laboratory techniques is the possibility of
using very small amplitude signals without
significantly disturbing the properties being
measured. To make an EIS measurement, a small
amplitude signal, usually a voltage between 5 to
50 mV, is applied to a specimen over a range of
frequencies of 0.001 Hz to 100,000 Hz. See how
our Sponsor can help you with these test
methods The EIS instrument records the real
(resistance) and imaginary (capacitance)
components of the impedance response of the
system. Depending upon the shape of the EIS
spectrum, a circuit model or circuit description
code and initial circuit parameters are assumed
and input by the operator. The program then fits
the best frequency response of the given EIS
spectrum, to obtain in fitting parameters. The
quality of the fitting is judged by how well the
fitting curve overlaps the original spectrum. By
fitting the EIS data it is possible obtain a set
of parameters which can be correlated with the
coating condition and the corrosion of the steel
substrate. Amongst the numerous equivalent
circuits that have been proposed to describe
electrochemical interfaces only the following
apply in the context of a freely corroding
interface at or close to kinetic equilibrium
Simplest RC representation of an electrochemical
interface One relaxation time constant with
extended diffusion Two relaxation time constants
Impedance of pitting processes of Al based
materials.
23
(No Transcript)
24
Model for a corroding paint on steel substrate
25
(No Transcript)
26
(No Transcript)
27
(No Transcript)
28
(No Transcript)
29
(No Transcript)
30
(No Transcript)
31
(No Transcript)
32
(No Transcript)
33
(No Transcript)
34
(No Transcript)
35
(No Transcript)
36
(No Transcript)
37
(No Transcript)
38
(No Transcript)
39
(No Transcript)
40
(No Transcript)
41
(No Transcript)
42
(No Transcript)
43
(No Transcript)
44
(No Transcript)
45
(No Transcript)
46
- Electrochemical NoiseElectrochemical Noise,
commonly abbreviated ECN, is an electrochemical
technique in which the potential and/or current
signals that arise directly from the
electrochemical reactions taking place on an
electrode surface are measured and interpreted.
ECN is of intense ongoing interest because of the
totally non-invasive nature of the measurement
when performed with a Zero Resistance Ammeter
(ZRA). The electrochemical instrument is not
applying any signal to the sample that might
improperly influence the result. This
perturbation may be of concern in every
electrochemical experiment other than noise. - ECN can also be measured with the
electrochemical instrument configured as a
potentiostat or galvanostat. The number of
actual applications of this sort is quite small.
Most ECN experiments are carried out with ZRA's,
so that no signal at all is applied to the sample
by the instrument.ECN has been used to monitor
localized corrosion (pitting), uniform corrosion
through measurement of the Noise Resistance, and
the deterioration of paints on metal substrates.
ECN does not yet enjoy the level of acceptance of
DC techniques and Electrochemical Impedance
Spectroscopy.
47
(No Transcript)
48
(No Transcript)