PV = nRT - PowerPoint PPT Presentation
Title:
PV = nRT
Description:
The density of a gas containing chlorine and oxygen has a density of 2.875 g/L ... Hint: The gas collected is a mixture so use Dalton's Law to calculate the ... – PowerPoint PPT presentation
Number of Views:643
Avg rating:3.0/5.0
Title: PV = nRT
1
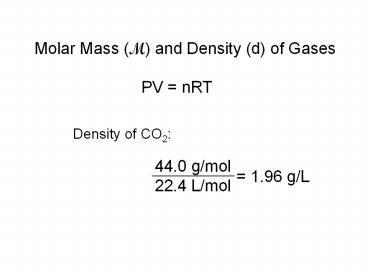
Molar Mass (M) and Density (d) of Gases
PV nRT
Density of CO2
44.0 g/mol 22.4 L/mol
1.96 g/L
2
Which gas would be the most dense? N2, CO2, He,
or O2
x g/mol 22.4 L/mol
d
3
How Molar Mass (M) and Density (d) are Related
PV nRT
P n RT V
MP nM RT V
d
Hint always use PV nRT first and watch your
units!
4
Practice
- An experiment shows that a 0.495 g sample of
an unknown gas occupies 127 mL at 98C and 754
torr pressure. Calculate the molar mass of the
gas.
5
Solution
(PV nRT)
6
Another Example
The density of a gas containing chlorine and
oxygen has a density of 2.875 g/L at 756 mmHg and
11oC. What is the most likely formula of the gas?
756 mmHg 0.995 atm 11oC 284 K
2.875 g/L
67.3g/mol
0.0427mol/L
PV nRT
n
(0.995atm)
(0.08206L.atm/mol.K)
(284K)
(V)
(0.995atm)
n
0.0427mol/L
(V)
(0.08206L.atm/mol.K)
(284K)
7
Daltons Law
- Gas identity is not important
- Mixture of gases obeys ideal gas law
- Dependent only on total number of moles
- Ptot P1 P2 P3
8
Daltons Law of Partial Pressures
- For a mixture of gases in a container
- PTotal P1 P2 P3
9
Mole Fraction
- Percentage of moles in a mixture
- Xi ni / ntot
- Pi XiPtot (partial pressure mole fraction x
total pressure)
10
Mole Fraction and Partial Pressure
C1 n1
P1 nTOTAL
PTOTAL
11
Mole Fraction Example
- At 25C, a 1.0 L flask contains 0.030 moles
of nitrogen, 150.0 mg of oxygen, and 4 x 1021
molecules of ammonia. - What is the partial pressure of each gas?
- What is the total pressure in the flask?
- What is the mole fraction of each?
12
Partial Pressures
13
Total Pressure
14
Mole Fractions
15
Mole Fractions
16
A sample of KClO3 is heated and decomposes to
produce O2 gas. The gas is collected by water
displacement at 25C. The total volume of the
collected gas is 229 mL at a pressure of 754
torr. How many moles of oxygen formed?
Practice
- Hint The gas collected is a mixture so use
Daltons Law to calculate the pressure of oxygen
then the ideal gas law to find the number of
moles oxygen.
PT PO2 PH2O
17
Solution