Wave Particle Duality - PowerPoint PPT Presentation
1 / 17
Title:
Wave Particle Duality
Description:
Wave / Particle Duality. PART I. Electrons as discrete Particles. Measurement of e (oil-drop expt. ... Compton Scattering: 'Particle-like' Behavior of Photon ... – PowerPoint PPT presentation
Number of Views:364
Avg rating:3.0/5.0
Title: Wave Particle Duality
1
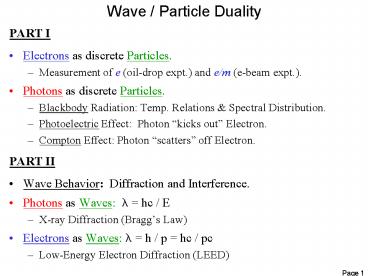
Wave / Particle Duality
- PART I
- Electrons as discrete Particles.
- Measurement of e (oil-drop expt.) and e/m (e-beam
expt.). - Photons as discrete Particles.
- Blackbody Radiation Temp. Relations Spectral
Distribution. - Photoelectric Effect Photon kicks out
Electron. - Compton Effect Photon scatters off Electron.
- PART II
- Wave Behavior Diffraction and Interference.
- Photons as Waves l hc / E
- X-ray Diffraction (Braggs Law)
- Electrons as Waves l h / p hc / pc
- Low-Energy Electron Diffraction (LEED)
2
Electrons Quantized Charged Particles
- In the late 1800s, scientists discovered that
electricity was composed of discrete or quantized
particles (electrons) that had a measurable
charge. - Found defined amounts of charge in electrolysis
experiments, where F (or Farad) NA e. - One Farad (96,500 C) always decomposes one mole
(NA) of monovalent ions. - Found charge e using Millikan oil-drop
experiment. - Found charge to mass ratio e/m using electron
beams in cathode ray tubes.
3
Electrons Millikans Oil-drop Expt.
- Millikan measured quantized charge values for oil
droplets, proving that charge consisted of
quantized electrons. - Formula for charge q used terminal velocity of
droplets fall between uncharged plates (v1) and
during rise (v2) between charged plates.
Charged oil droplets
Charged Plates
Scope to measure droplet terminal velocity.
4
Electron Beam e/m Motion in E and B Fields
Circular Motion of electron in B field
? Larger e/m gives smaller r, or larger
deflection.
Electron (left hand)
Proton (right hand)
5
Electron Beam e/m Cathode Ray Tube (CRT)
- Tube used to produce an electron beam, deflect it
with electric/magnetic fields, and then measure
e/m ratio. - Found in TV, computer monitor, oscilloscope, etc.
J.J. Thomson
Charged Plates (deflect e-beam)
Deflection ? e/m
() charge
Cathode (hot filament produces electrons)
() charge
Slits (collimate beam)
Fluorescent Screen (view e-beam)
6
Ionized Beam q/m Mass Spectrometer
- Mass spectrometer measures q/m for unknown
elements.
1.
Ions accelerated by E field.
Ion path curved by B field.
2.
2.
1.
7
Photons Quantized Energy Particle
- Light comes in discrete energy packets called
photons.
Energy of Single Photon
Rest mass
From Relativity
For a Photon (m 0)
Momentum of Single Photon
8
Photons Electromagnetic Spectrum
400 nm
Gamma Rays
X-Rays
Ultraviolet
Visible Spectrum
Visible
Frequency
Wavelength
Infrared
Microwave
Short Radio Waves
TV and FM Radio
AM Radio
Long Radio Waves
700 nm
9
Photoelectric Effect Particle Behavior of
Photon
PHOTON IN ? ELECTRON OUT
- Photoelectric effect experiment shows quantum
nature of light, or existence of energy packets
called photons. - Theory by Einstein and experiments by Millikan.
- A single photon can eject a single electron from
a material only if it has the minimum energy
necessary (or work function f). - For example, if 1 eV is necessary to remove an
electron from a metal surface, then only a 1 eV
(or higher energy) photon can eject the electron.
10
Photoelectric Effect Particle Behavior of
Photon
PHOTON IN ? ELECTRON OUT
- Electron ejection occurs instantaneously,
indicating that photons cannot be added up. - If 1 eV is necessary to remove an electron from a
metal surface, then two 0.5 eV photons cannot add
together to eject the electron. - Extra energy from the photon is converted to
kinetic energy of the outgoing electron. - For example above, a 2 eV photon would eject an
electron having 1 eV kinetic energy.
11
Photoelectric Effect Apparatus
- Photons hit metal cathode and eject electrons
with work function f. - Electrons travel from cathode to anode against
retarding voltage VR(measures kinetic energy Ke
of electrons).
- Electrons collected as photoelectric current at
anode. - Photocurrent becomes zero when retarding voltage
VR equals stopping voltage Vstop, i.e. eVstop
Ke
Cathode
Anode
Light
12
Photoelectric Effect Equations
- Total photon energy e ejection energy e
kinetic energy. - where hc/l photon energy, f work function,
and eVstop stopping energy. - Special Case No kinetic energy (Vo 0).
- Minimum energy to eject electron.
13
Photoelectric Effect IV Curve Dependence
Intensity I dependence
Vstop Constant
f1 gt f2 gt f3
Frequency f dependence
f1
f2
f3
Vstop? f
14
Photoelectric Effect Vstop vs. Frequency
hfmin
Slope h Plancks constant
-f
15
Photoelectric Effect Threshold Energy Problem
If the work function for a metal is f 2.0 eV,
then find the threshold energy Et and wavelength
lt for the photoelectric effect. Also, find the
stopping potential Vo if the wavelength of the
incident light equals 2?t and ?t /2. At
threshold, Ek eVo 0 and the photoelectric
equation reduces to
For 2?t, the incoming light has twice the
threshold wavelength (or half the threshold
energy) and therefore does not have sufficient
energy to eject an electron. Therefore, the
stopping potential Vo is meaningless because
there are no photoelectrons to stop! For ?t/2,
the incoming light has half the threshold
wavelength (or twice the threshold energy) and
can therefore eject an electron with the
following stopping potential
16
Compton Scattering Particle-like Behavior of
Photon
- An incoming photon (E1) can inelastically scatter
from an electron and lose energy, resulting in an
outgoing photon (E2) with lower energy (E2 lt
E1). - The resulting energy loss (or change in
wavelength Dl) can be calculated from the
scattering angle q.
Incoming X-ray
Scattered X-ray
Scattering Crystal
Angle measured
17
Compton Scattering Schematic
PHOTON IN ? PHOTON OUT (inelastic)