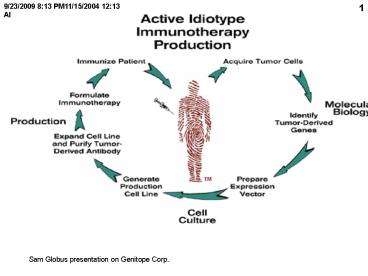
Sam Globus presentation on Genitope Corp. - PowerPoint PPT Presentation
1 / 50
Title:
Sam Globus presentation on Genitope Corp.
Description:
Wang et al.: double K.O. of splicing factor gene; strategy, coverage of viability ... in cultured lines: myc double K.O. = viable, sick (Skip?) 23. Or (50:50) ... – PowerPoint PPT presentation
Number of Views:110
Avg rating:3.0/5.0
Title: Sam Globus presentation on Genitope Corp.
1
Sam Globus presentation on Genitope Corp.
2
Papers
- Kohler and Milstein hybridoma, classic,
methods, result - Littlelfield cell hybridization, classic, method
- WIgler et al (1980) co-transfection and
co-amplification, classic, phenomenon - Tanaka et al. possible gen amp. mech.,
mechanism - Evans et al., cDNA gene identification cloning,
method - Wang et al. double K.O. of splicing factor gene
strategy, coverage of viability
3
- Categories of cell mutants
- Exploitable metabolic pathways
- Purine and pyrimidine biosynthesis auxotrophs
- (auxotrophs require a nutrient in the medium that
the WT doesnt) - Amino acid biosynthesis auxotrophs
- Auxotrophs BUdR (BrdU) Kao and Puck. Kill
growing cells. Penicillin analogy. - Amino acid, nucleotide biosynthetic
pathways. - 2. Drug resistance see sheet
- A. Mutant lacks toxifying enzyme e.g., HPRT
(TGR), APRT (DAPR, 8-azaAR), TK (BrdUR) - B. Enzyme target becomes a better discriminator
(ouabain NaK ATPase pump) - C. Permeation changes influx blocked or efflux
increased. (MDR, P-glycoprotein) - D. Improved de-toxification via chelation,
covalent modification, - or overproduction of target (dhfr
MTX-resistance) - E. Receptor deficiency
- Glucocorticoid (steroid) receptor-resistant to
GC killing of lymphoid lines.
4
(No Transcript)
5
(No Transcript)
6
(No Transcript)
7
- 3. Temperature-sensitive mutants cell cycle
mutants. - Tritiated amino acid suicide (aa-tRNA
synthetases) - 4. Antibodies. Lysis with complement. Targets
cell surface constituents mostly (e.g., MHC) - 5. Visual inspection at colony level
- A. Sib selection (G6PD)
- B. Replica plating (LDH)
- C. Secreted product (Iganti-Ig IP)
- FACS fluorescence-activated cell sorter. 1-D
and 2-D fluorescence displays (cell surface Ag) - Brute force (clonal biochemical analysis, e.g.,
electrophoretic variants (e.g., Ig, isozymes) - Direct genotype analysis (rare) (DNA isolation
(via PCR and SSCP, single strand conformational
polymorphism electrophoresis. Or DGGE denaturing
gradient gel electrophoresis. - MHC major histocompatability locus or proteins
G6PD glucose-6-phosphate dehydrogenase LCH
lactate dehydrogenase Ig immunoglobulin
8
Cell fusion (for gene juxtaposition, protein
trafficking, mapping) Fusogenic agents PEG,
Sendai virus (syncytia promoting, as
HIV). Heterokaryons (2 nuclei), no cell
reproduction (limited times). (e.g., membrane
fluidity, nuclear shuttling, gene activation
(myoblasts) Hybrids (nuclei fuse, cells
reproduce). Complementation (e.g., auxotrophs
with same requirement) Dominance vs.
recessiveness. Mapping chromosome assignment.
Synteny. Radiation hybrids linkage analysis
9
Cell fusion
Hprt, TK-
Parental cells
Hprt-, TK
HAT-
HAT-
PEG (polyethylene glycol, mw 6000 Sendai virus,
inactivated
Cell fusion
Heterokaryon (or, alternatively, homokaryon)
HAT medium
Hprt-, TK, Hprt TK-
HAT
Cell cycle, Nuclear fusion, Mitosis, survival
Hprt-, TK, Hprt TK-
membrane dynamics (lateral diffusion
Edidin), shuttling proteins (hnRNP A1
Dreyfuss), gene regulation (turn on myogenesis
Blau)
Hybrid cell
gene mapping (synteny Ruddle) gene regulation
(extinction Weiss) Complementation (pyrimidine
path Patterson)
10
Complementation analysis
Parental cells
Parental cells
gly-
gly-
gly-
gly-
Cell fusion
Cell fusion
glyA- glyA-
glyA- glyB-
Hybrid cell
Hybrid cell
Glycine-free Medium No growth, no
complementation, ?same gene (named glyA)
Glycine-free Medium Yes, growth, Yes,
complementation, ?different genes genes (named
glyA and glyB)
11
Hybrid cell
Reduced hybrid
Spontaneous chromosome loss (human
preferentially)
Hprt-, TK, Hprt TK-
Hprt-, TK, Hprt TK-
Correlate identified chromosome loss With loss of
phenotypic trait (isozyme, DNA sequence, etc.)
12
Mary Weiss Mapping, liver gene regulation
Frank Ruddle (mapping)
Helen Blau (myogenesis)
Henry Harris (cancer, differentiation)
13
Sub-chromosomal mapping using radiation hybrids
14
(of clones)
Correlate sequence tag with phenotypic trait
(isozyme,etc.)
15
Transfection
Transfection agents DEAE-dextran (toxic, OK for
transient) CaPO4 (co-precipitate) Electroporation
(naked DNA, high quick voltage ? transient
holes) Lipofection (multilamellar
liposomes) Polybrene (detergent?) Ballistic
(DNA-coated gold particles) Must traverse
cytoplasm. Much engulfed in lysosomes.
Inhibition of lysosomal function often helps
(chloroquin) Pechelosome 2000 KB
co-integration of high MW DNA. Separate plasmids
-gt same site (co-integration). Separate
transfections -gt separate locations Random or
semi-random (many) integration sites (unless
targeted) Low but real homologous recombination
rate
16
Mike Wigler
Richard Axel
Saul Silverstein
History discovered for practical use at Columbia
(PS Wigler Axel and Silverstein)
17
Transient transfection vs.
permanent cloned genes Unintegrated DNA
chromosomally
integrated Unnatural?
position effects
? Super-physiological expression (so
average many) levels (per transfected cell)
? Transient -gt 10-50 transfection efficiency
(stain) Permanents more like 0.001 per µg DNA
per cell (high). i.e., 106 -gt 1000 colonies
could be much less for certain types of cells
18
(skip)
One the most dramatic first applications of gene
transfection from total DNA Transfer of the
growth-transformed phenotype ability to grow in
multilayers or in suspension in soft agar
(Weinberg, Wigler) DNA from tumor transfected
into growth controlled mouse 3T3 cells. Look
for foci (focus). Make a library from
growth-transformed transfectant. Screen for human
Alu repeat. Verify cloned DNA yields high
frequency of focus-forming transfectants. Isolate
cDNA by hybridization. Sequence. Identify gene
a dominant oncogene. Ras, a signaling protein
in transducing pathway for sensing growth factors
19
Gene discovery Use transfection to clone new
genes Transfect genomic or cDNA libraries into
mutant or WT cells. e.g Tumor DNA normal
cells ----gt foci or growth in agar, oncogene
ID WT DNA DNA repair deficient mutant ----gt
repair gene ID WT cDNA from cell type 1 WT
cells type 2 ----gt receptor gene, e.g.
20
Edwards et al. paper Cloning the cDNA for an
opioid receptor Footnote 10 on the method
CDM8 COS cells Hirt extraction 125 I
21
Transfection
Transient transfection Cells ? die
22
(Skip?)
Recombination gene targeting Mitotic
recombination between homologous chromosomes
relation to cancer through the loss of tumor
suppressor genes LOH loss of homozygosity WT
/ ? mutation ? /- (WT phenotype) ? LOH
via homologous recombination, or loss and
duplication ? -/- Recombination of transfected
genes homologous vs. non-homologous
recombination. Gene conversion vs. reciprocal
recombination. Recombination between tandem
inserts (higher freq) Gene knockouts via
homologous recombination. ES cells and
transgenic mice. Selection for homologous
recombinants via the loss of viral TK genes
(Capecchi). Allele replacements in cultured cell
lines (e.g., APRT). Little work in cultured
lines myc double K.O. viable, sick
23
(Skip?)
Loss of heterozygosity (LOH) by mitotic
recombination between homologous chromosomes
(rare)
-
-
(This is not sister chromatid exchange)
1 homozygote 1 homozygote -
Or (5050)
-
-
-
-
Heterozygote
After homologous recombination
-
-
-
Also chromosome loss reduplication
double mutation
2 heterozygotes again
24
Gene amplification Historically Methotrexate
resistance (Littlefield) High dihydrofolate
reductase (DHFR) enzyme activity DHFR protein
protein synthetic rate translatable mRNA mRNA
level (Schimke) DNA level (no. of
genes) Homogeneously staining, expanded
chromosomal regions (HSRs) Biedler Nunberg
HSR dhfr genes. (ISH) Double minute
chromosomes Amplicons. Big (300 KB). Can shrink,
migrate.
25
(No Transcript)
26
Gene amplification
Homogeneously staining region FISH, here
27
HSR ? dmin upon DS break induced by a homing
endonuclease (I-SceI).
HSR homogeneously staining region Dmin double
minute chromosomes
Arnaud Coquelle, Lorène Rozier, Bernard
Dutrillaux and Michelle Debatisse ONCOGENE, 31
October 2002, Volume 21, Number 50, Pages
7671-7679 Induction of multiple double-strand
breaks within an hsr by meganucleaseI-SceI
expression or fragile site activation leads to
formation of double minutes and other chromosomal
rearrangements
28
Ampification modelsOver-replication, unequal
sister chromatid exchange, breakage and
fusion. In nature rDNA in oocytes,
Drosophila chorion genes. In medicine
chemotherapy resistance (MDR, P-glycoprotein,
efflux pump) cancer (myc, ras) In
biotechnologyhigh level recombinant protein
production in mammalian cells
29
Fred Alt
Geoff Wahl
George Stark
30
Tanaka et al.
31
(No Transcript)
32
Gene amplification for high level production in
CHO dhfr- cells.
33
Reduction of folate to tetrahydrofolate
34
(No Transcript)
35
Biosynthesis of glycine
36
Biosynthesis of TMP
37
Biosynthesis of purine nucleotides
38
DHFR- cells require G,H,T
39
Transfection strategies
- YFG linked to a dhfr minigene on a single plasmid
- A. Insures co-integration
- B. Insures co-amplification
- YFG and dhfr on separate plasmids
- A. Allows a high ratio of YFG to dhfr to start
40
Linked amp
CHO cells
41
Co-amp1
42
Co-amp3
43
kaufman
44
Co-amp2
45
Co-amp4
46
Amplification protocol
47
A different major system for high level Mab
production NS0 cells Mouse myeloma cells, high
IgG producers ? IgG variants NS0 No endogenous
IgG, but cell is a natural IgG secretor. Lack
glutamine synthetase (GS) glutamate NH3
ATP ? glutamine ADP Pi Vector MAb genes
driven by strong promoters (H-chain, L-chain)
GS cDNA gene (Bebbington) Select on
glutamine-free medium Inhibit GS with methionine
sulfoximine (gln analog) Select for GS
overproducers ---gt--gt (amplification of the GS
cDNA gene and linked Mab genes) Proprietary
(Lonza Biologics)
48
Some other amplifiable genes
49
Position effect (Reff- IDEC) Expression level is
influenced by the position of integration (in
transgenic mice and transfected cells
) Euchromatin vs. heterochromatin, gradation,
proximity of enhancers Reff Screen for a high
producer site among many transfectants. Integrate
d gene is linked to 1/3 of a neo gene (3 exons),
and several selectable markers including dhfr
(amplifiable) . Use this transfectant as the
host for YFG linked within (why?) the other 2/3
of the neo gene. Overlapping neo sequences
target homologous recombination. Select for G418
resistance (reconstruction of the neo
gene) Drug-resistant colonies carry YFG at the
hot spot for production,within an intron of the
neo gene. Homologous recombination frequency is
low (10-7), but you only need one good
transfectant. Amplify with MTX / dhfr.
50
CMV-MCS
dhfr
lucD
GUS
HisD
neo3
Marker, integrated first.
neo1
neo2
CD20
Targeting plasmid