Spectroscopy
1 / 115
Title: Spectroscopy
1
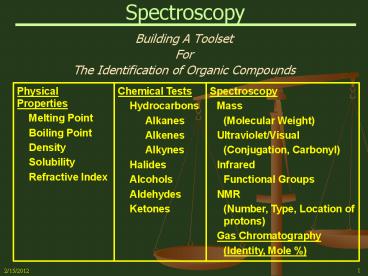
Spectroscopy
- Building A Toolset
- For
- The Identification of Organic Compounds
2
Spectroscopy
- Spectroscopy Tools
3
Spectroscopy
- Spectroscopy
- The Absorption of Electromagnetic Radiation and
the use of the Resulting Absorption Spectra to
Study the Structure of Organic Molecules. - When continuous radiation passes through a
transparent material, some of the radiation can
be absorbed. - If the portion that is not absorbed is passed
through a prism, a spectrum with gaps is
produced. - This is called an
- ABSORPTION SPECTRUM
4
Spectroscopy
- Energy States
- Energy absorption by transparent materials in any
portion of the electromagnetic spectrum causes
atoms or molecules to pass from a state of low
energy (ground state) to a state of higher energy
(excited state). - There are 3 types of Energy States
- Electronic
- Vibrational
- Spin
5
Spectroscopy
- Electromagnetic Spectrum
- Cosmic ? (Gamma) X-Ray
- Ultraviolet Visible Infrared
- Microwave Radio
- Energy States and the Electromagnetic Spectrum
- Electronic Ultraviolet
- Vibrational Infrared
- Spin Radio
6
Spectroscopy
3 x 108 Hz
1.2 x 1014 Hz
Frequency
3 x 1019 Hz
3 x 1016 Hz
2 x 1013 Hz
6 x 107 Hz
1.5 x 1015 Hz
1 x 109 Hz
3 x 1011 Hz
4 x103cm-1
1.25 x104cm-1
Wave Number
0.002 cm-1
2.5 x104cm-1
0.01 cm-1
1 x109cm-1
10 cm-1
3 cm-1
1 x107cm-1
5 x104cm-1
667cm-1
Cosmic ? Ray
Vacuum UV
Microwave
Infrared
X-Ray
Radio
Frequency
1 m
5 m
0.01 nm
10 nm
30 cm
1 mm
Wavelength
400 nm
200 nm
800 nm
2.5 ?
15 ?
Visible
Near Ultraviolet
Nuclear Magnetic Resonance
Vibrational Infrared
Blue
Red
7
Spectroscopy
- Quantization
- The excitation process is quantized, in which
only selected frequencies of energy are absorbed
representing the energy difference (?E) between
the excited and ground states.
?E E (excited) E (ground) h?
E hc / ?
? c / ?
Where
? Frequency (Hz) c Velocity of Light
(cm/sec) ? Wavelength (cm) h Plancks
Constant
8
Spectroscopy
- Spectroscopy Types
- Mass Spectrometry (MS) Hi-Energy Electron
Bombardment - Use Molecular Weight, Presence of Nitrogen,
Halogens - Ultraviolet Spectroscopy (UV) Electronic Energy
States - Use Conjugated Molecules Carbonyl Group, Nitro
Group - Infrared Spectroscopy (IR) Vibrational Energy
States - Use Functional Groups Compound Structure
- Nuclear Magnetic Resonance (NMR) Nuclear Spin
States - Use The number, type, and relative position of
protons (Hydrogen nuclei) and Carbon-13
nuclei
9
Mass Spectroscopy
- High energy electrons bombard organic molecules
breaking some or all of the original molecules
into fragments. - The process usually removes a single electron to
produce a positive ion (cation radical) that can
be separated in a magnetic field on the basis of
the mass / charge ratio. - Removal of the single electron produces a charge
of 1 for the cation. - Thus, the cation represents the Molecular Weight
of the original compound or any of the fragments
that are produced. - The mass spectrum produced is a plot of relative
abundance of the various fragments (positively
charged cation radicals) versus the Mass / Charge
(M/Z) ratio. - The most intense peak is called the Base Peak,
which is arbitrarily set to 100 abundance all
other peaks are reported as percentages of
abundance of Base Peak.
10
Mass Spectroscopy
M e- ? M
2e-
Molecule
High Energy Electron
Molecular Ion (Radical Cation)
1-Pentanol - MW 88CH3(CH2)3 CH2OH
M - (H2O and CH2 CH2)
Base Peak
M - (H2O and CH3)
Typical Mass Spectrum
M - H2O
CH2OH
Molecular Ion Peak (M 88)
11
Mass Spectroscopy
- Molecular Ion Peak (M)
- Largest mass/charge ratio
- Always the last peak on the right side of
spectrum - May or may not be the base peak (usually not)!
- Abundance can be quite small, i.e., very small
peaks - The Molecular Ion Peak represents the
- Molecular Weight of the Compound
12
Mass Spectroscopy
Methyl Propyl Ketone (C5H10O) (CAS 107-87-9)
M 43(C2C2CH3) lostPropyl Group
M 28(CH2CH2) lost
M 15(CH3) lost
M 86
13
Mass Spectroscopy
- The Presence of Nitrogen in the Compound
- If the Mass / Charge (m/z) ratio for the
Molecular Ion peak is Odd, then the molecule
contains an Odd number of Nitrogen atoms, i.e.,
1, 3, 5, etc. - Note An Even value for the Mass / Charge
ratio could represent a compound with an even
number of Nitrogen atoms, i.e., 0, 2, 4 etc. - The actual presence of Nitrogen in the compound
is not explicitly indicated as it is with an
Odd value for the ratio.
14
Mass Spectroscopy
- Halogens in Organic Compounds
- Most elements exist in several isotopic forms
- Ex. 1H1, 2H1, 12C6, 13C6, 35Cl17, 37Cl17,
79Br35, 81Br35 - Average Molecular Weight
- The average molecular weight of All isotopes of
a given element relative to the abundance of the
each isotope in nature - Integral Molecular Weight
- The Number of Protons and Neutrons in a specific
isotope - Each fragment represented in a Mass Spectrum
produces several peaks each representing a
particular isotopic mixture of the elements in
the compound, i.e., an integral molecular weight.
15
Mass Spectroscopy
- The Presence of Chlorine in a Compound
- The two (2) principal Chlorine Isotopes in nature
areCl-35 and Cl-37 (2 additional Neutrons in
Cl-37) - The relative abundance ratio of Cl-35 to Cl-37
is - 100 32.6 or 75.8 24.2 or ? 3 1
- Therefore, a Molecule containing a single
Chlorine atom will show two Mass Spectrum
Molecular Ion peaks, one for Cl-35 (M) and one
for Cl-37 (M2) - Note M2 denotes 2 more neutrons than M
- Based on the natural abundance ratio of 100 /
32.6 (about 31), the relative intensity (peak
height) of theCl-35 peak will be 3 times the
intensity of the Cl-37 peak
16
Mass Spectroscopy
- The Presence of Chlorine in a Compound (Cont)
1-Chloropropane
Molecule contains 1 Chlorine atom resulting in
two Molecular Ion Peaks of about 31 relative
intensity, based on the 31 natural abundance
ratio ofCl-35 / Cl-37
Molecular Ion Peaks M 78 M2 80
very small
17
Mass Spectroscopy
- The Presence of Bromine in a Compound
- The two (2) principal Bromine Isotopes in nature
areBr-79 and Br-81 (2 additional Neutrons in
Br-81) - The relative abundance ratio of Br-79 to Br-81 is
- 100 97.1 or 50.5 49.5 or ? 1 1
- Molecules containing a single Bromine atom will
also show two molecular ion peaks one for Br-79
(M) and one for Br-81 M2 - Based on the natural abundance ratio of 100 /
97.1 (about 11), the relative intensity of the
Br-79 peak will be about the same as the Br-81
peak
18
Mass Spectroscopy
- The Presence of Bromine in a Compound (Cont)
19
Mass Spectroscopy
- The Presence of Fluorine in a Compound
- Fluorine exists in nature principally as a single
isotope 19F9 - A compound containing any number of Fluorine
atoms will have a single Molecular Ion peak
(assuming no other Halogens present)
20
Mass Spectroscopy
- Multiple Halogens in a Compound
- Compounds containing two (2) Chlorine atoms will
produce three (3) Molecular Ion peaks
representing the 3 possible isotope combinations
of Chlorine - 35Cl17 35Cl17 (Rel Peak Intensity - 100.0)
- 35Cl17 37Cl17 (Rel Peak Intensity -
65.3) - 37Cl17 37Cl17 (Rel Peak Intensity -
10.6)
21
Mass Spectroscopy
- Multiple Halogens in a Compound
- Compounds containing three (3) Chlorine atoms
will produce four (4) Molecular Ion peaks
representing the 4 possible isotope combinations
for Chlorine - 35Cl17 35Cl17 35Cl17 (Rel Peak
Intensity - 100.0) - 35Cl17 35Cl17 37Cl17 (Rel Peak
Intensity - 97.8) - 35Cl17 37Cl17 37Cl17 (Rel Peak
Intensity - 31.9) - 37Cl17 37Cl17 37Cl17 (Rel Peak Intensity -
3.5)
22
Mass Spectroscopy Molecular Formula
- Information from the Mass Spectrum can used to
determine the Molecular Formula of a compound - Ex. Molecular Ion Peaks M 94 M2 96 (95)
- 2 Molecular Ion Peaks (31) suggest 1
Chlorine atom - Partial Analysis C 25.4 H 3.2
- Use 95 as average molecular weight
- Carbon 95 x 0.254 24.1 / 12 2 C atoms
- Hydrogen 95 x 0.032 3.0 / 1 3 H
atoms - 95 (24 3) 68 unresolved mass
- (Use oxygen, nitrogen, halides (Cl or Br) to
resolve mass) - 2 Oxygen (16 16) 1 Chlorine (35.5) ? 68
- Molecular Formula - C2H3O2Cl
23
Mass Spectroscopy
- Summary
- Fragmentation of Organic Molecules by high energy
electrons - Base Peak 100 abundance
- Molecular Ion Peak Highest Mass/Charge ratio
- Molecular Ion Peak Last peak(s) on right side
of chart - Molecular Ion Peak Represents Molecular Weight
of compound - Molecular Ion Peak If value is Odd the
compound contains an odd number of Nitrogen
atoms - Molecular Ion Peak If two peaks occur and the
relative abundance ratio is 31, then the
compound contains a single Chlorine atom. - Molecular Ion Peak If two peaks occur and the
relative abundance ration is 11, then the
compound contains a single Bromine Atom
24
Ultraviolet/Visible (UV) Spectroscopy
- UV-Visible Spectrum 190 nm 800 nm
- In Ultraviolet and Visible Spectroscopy, the
energy absorption transitions that occur are
between electronic energy levels of valence
electrons, that is, orbitals of lower energy are
excited to orbitals of higher energy. - Thus, UV / Visible spectra often called
Electronic Spectra - All organic compounds absorb Ultraviolet light to
some degree, but in many cases at such short
wavelengths to make its utility of very limited
value in organic chemistry. - For the purpose of this course, the primary use
of UV/Vis will be to confirm - The presence of conjugated molecules, either
aliphatic alkene structures or aromatic ring
structures. - To a lesser degree, the presence of the Carbonyl
group and the Nitro group.
25
Ultraviolet/Visible (UV) Spectroscopy
- When a molecule absorbs radiation a valence
electron is generally excited from its highest
occupied molecular orbital (HOMO) to the lowest
unoccupied molecular orbital (LUMO) - For most molecules, the lowest energy orbitals
are thesigma (?) orbitals (? - bonds) - The electrons of sigma bonds are too tightly
bound to be promoted by radiation in the 200-700
nm region. - Therefore alkanes, saturated alcohols, simple
alkenes show no or very little UV absorption. - The ? orbitals occupy somewhat higher energy
levels. - Orbitals that hold unshared pairs of electrons,
the nonbonding (n) orbitals, lie at even higher
energies. - Unoccupied or antibonding orbitals (? and ?)
have the highest energies.
26
Ultraviolet/Visible (UV) Spectroscopy
- Chromophores
- The absorption of Ultraviolet radiation results
from the excitation of electrons from ground to
excited state - The Nuclei in molecules, however, determine the
strength with which electrons are bound to the
molecule, thus, influencing the spacing between
ground and excited states - The characteristic energy of a transition and the
wavelength of radiation absorbed are properties
of a group of atoms rather than the electrons
themselves. - The group of atoms producing such an absorption
is called a Chromophore. - As the structure of the molecule (alkane, alkene,
alkyne, alcohol, amine, nitrile, carbonyl, etc.)
changes, the energy and intensity of the
Ultraviolet absorption will change accordingly
27
Ultraviolet/Visible (UV) Spectroscopy
- Radiation in the range 190nm 800nm causes
valence electrons (those that participate in
bonding) to be excited to a higher energy level. - The ground state of an organic molecule can
contain valence electrons in three principal
types of molecular orbitals
? (sigma) ? (pi) n (non-bonding)
Sigma pi bonds have antibonding
(unocuupied) orbitals associatedwith them
? ?
28
Ultraviolet/Visible (UV) Spectroscopy
Energy Transition Examples
- n ? ? in carbonyl compounds
- n ? ? in oxygen, nitrogen, sulfur, and halogen
compounds - ? ? ? in alkenes, alkynes, carbonyl and azo
compounds - ? ? ? in carbonyl compounds
- ? ? ? in alkanes
IncreasingEnergy
- ?
- n
- ?
- ?
Antibonding (single bonds) Antibonding (double
bonds)
IncreasingEnergy
Nonbonding (as in lone electron pairs or the
propenyl (allyl) radical
Bonding (double bonds) Bonding (single bonds)
Note Electronic energy levels in aromatic
molecules are more complicated than are presented
here.
29
Ultraviolet/Visible (UV) Spectroscopy
- Atoms produce sharp lines of absorption
- Molecules have many excited modes of vibration
and rotation at room temperature. The rotational
and vibrational levels are superimposed on the
electronic levels - Electron transitions may occur from any of
several vibrational and rotational states of one
electronic level to any of several vibrational
and rotational states of a higher electronic
level. - Thus, the UV spectrum of a molecule consists of a
broad band of absorption centered near the
wavelength of the major transition, i.e. where
the radiation has its maximum absorption (?max).
30
Ultraviolet/Visible (UV) Spectroscopy
- The Ultraviolet-Visible spectrum is generally
recorded as a plot of absorbance versus
wavelength but the plot is more often presented
with the Absorptivity (?) or log ? plotted on the
ordinate (y-axis) and the wavelength plotted on
the abscissa (x-axis) - Ex Cyclohexane
- (A Conjugated Aromatic Molecule)
- Wavelength of Maximum Absorbance ?max 230 nm
- Molar Absorptivity
- ? 15,000 cm-1
- Log ? 4.2
31
Ultraviolet/Visible (UV) Spectroscopy
- The Beer-Lambert Law
- The Ultraviolet/Visible Spectrum is a plot of the
Wavelength (?) in nanometers (nm) over the entire
Ultraviolet / Visible region versus the
Absorbance (A) of the radiation at each
wavelength. - A log (Ir / Is) ? C L
- Is Intensity of light through sample solution
- Ir Intensity of incident light passing through
Reference cell - ? Molar Absorptivity (Molar Extinction
Coefficient) A measure of the strength or
intensity of the absorption. - Units l/(mol cm) (m2 x 10-2 /mol)
(mmol/dm3) - C Concentration of solute (mol/L) or (g/L) if
molecular mass is unknown - L Length of cell (cm)
32
Ultraviolet/Visible (UV) Spectroscopy
- A ? C l
- ? A / (C l )
- Values of ? are usually expressed as Log ?
- Aliphatic (single band) ? 10,000 20,000 (Log
? 4.0 4.3) - Aromatic (two bands ? 1,000 10,000 (Log ?
3.0 4.0) - Carbonyl compounds ? 10 100 (Log ? 2)
- Nitro compounds ? 10 (Log ? 1)
33
Ultraviolet/Visible (UV) Spectroscopy
- Typical Transitions Associated Wavelengths of
Maximum Absorption and Molar Absorptivities
34
Ultraviolet/Visible (UV) Spectroscopy
- Typical Transitions and Absorptionsof Simple
IsolatedChromophores
35
Ultraviolet/Visible (UV) Spectroscopy
- Computation Example
- An ??-unsaturated ketone of relative molecular
weight 110 has an absorption band with ?max at
215 nm and ? 10,000 (l / mol cm) - A solution of this ketone showed absorbance A
2.0 with a 1 cm cell. Calculate the concentration
of the ketone in this solution expressed in grams
per liter. - Ans A ? c L
- c A / ? L
- c 2.0 / ((10,000 l/mol cm) 1.0 cm)
- c 2 x 10-4 mol/l
- c 2 x 10-4 mol/l x 110 g/mol
- c 2.20 x 10-2 g/l
36
Ultraviolet/Visible (UV) Spectroscopy
- Computation Example
- Calculate the Molar Absorptivity, ?, for a
solution containing 1.0 mmol dm-3 (1.0 x 10-3
moles per liter) of solute, when the absorbance
of a 1 cm cell was 1.5. - Ans A ? c L
- ? A / c L
- ? 1.5 / (1 x 10-3 mol / L) (1 cm)
- ? 1.5 x 103 L / mol cm
- What would be the Absorbance for a solution of
double this concentration? - Ans A 1.5 x 103 L / mol cm 2.0 x 10-3
moles / L 1 cm - A 3.0
37
Ultraviolet/Visible (UV) Spectroscopy
- Alkanes
- Contain single sigma bonds resulting in only ? ?
? transitions which absorb ultraviolet radiation
at wavelengths generally too short for use in UV
spectroscopy. - Utility None
- Alcohols, Ethers, Amines, Sulfur Compounds
- The n ? ? transitions absorb UV radiation within
the experimentally accessible range (gt180 nm). - Utility Very little
38
Ultraviolet/Visible (UV) Spectroscopy
- Alkenes and Alkynes
- Absorb UV radiation in the range lt 180 nm.
- Cumulated alkenes (? ? ? transitions), which
have one or more ? sigma bonds between the
double bounds usually have absorption maxima
below 200 nm. - Utility Very little
- Compounds with Oxygen double bonds
- Unsaturated molecules containing oxygen or
nitrogen structures such as Carbonyl (CO) and
Nitro (NO2) have both n ? ? (280 - 290 nm) and ?
? ? transitions (188 nm). - Utility Moderate
39
Ultraviolet/Visible (UV) Spectroscopy
- Conjugated unsaturated systems are molecules with
two or more double or triple (?) bonds each
alternating with a single or sigma bond (?). - Conjugated unsaturated systems have delocalized ?
bonds, i.e., a p-orbital on an atom adjacent to a
double bond producing ? ? ? transitions. - Single electron as in the allyl radical
(CH2CH?CH2) - Vacant p orbital as in allyl cation
(CH2CH?CH2) - P orbital of another double bond
(CH2CH?CHCH2 - Conjugated systems include the Aliphatic Alkenes
as well as the Aromatic ring structures. - Compounds whose molecules contain conjugated
multiple bonds absorb strongly in the UV /
Visible portion of the electromagnetic spectrum
(gt 200 nm). - Utility Good
40
Ultraviolet/Visible (UV) Spectroscopy
- Conjugated Unsaturated Systems
- Conjugated systems consist of alternating sigma
(?) bonds and pi (?) bonds) and the Ultraviolet
absorptions show large values of ?
2,5-Dimethyl-2,4-Hexadiene (in Methanol)
- The Wavelength of Maximum Absorption ( ?max ) is
obtained from the Absorption Spectrum - Wavelength of Maximum Absorbance (?max) 242.5
nm - Molar Absorptivity ( ? ) 13,100 M-1 cm-1 (Log
? 4.1)
41
Ultraviolet/Visible (UV) Spectroscopy
- Conjugated Unsaturated Systems (Cont)
- ?,? - Unsaturated ketones, Dienes, Polyenes
- Transitions ? - ?
- High Intensity Bands
- ? 10,000 to 20,000 (log ? 4.0 - 4.3)
- ?max gt 210 nm
- Aromatic Conjugated Systems
- Transitions ? - ?
- 2 Medium Intensity Bands
- ? 1000 - 60,000 (log ? 3.0 - 4.8)
- ?max both bands gt 200 nm
- Note Substitution on ring increases Molar
Absorptivity above 10,000
42
Ultraviolet/Visible (UV) Spectroscopy
- Carbonyl (CO), Nitro Group (NO2) (Resonance
effects on substituted benzene) - Transitions n - ? ? ? ?
- Single Low Intensity Band ? 10 (log ? 1)
to ? 300 (log ? 2.5) - ?max (250 - 360 nm)
- Nitro (NO2) log ? (1.0)
- Carbonyl (CO) log ? (2.0)
- The presence of these functional groups should be
used only as confirmations of species identified
in the IR Spectra.
43
Ultraviolet/Visible (UV) Spectroscopy
- Practical Approach to Interpreting UV/Vis
Information - If the problem you are working on provides an
UV/Vis spectrum and it indicates No absorption
in the 200 700 nm range, the following
conclusions are applicable - The compound is not conjugated, i.e., it does not
contain alternating double/single bonds
(including Benzene ring.) - The compound probably does not contain
Carbonyl or Nitro groups (confirm with IR). - If the problem provides Log Absorptivity values
(Log ?) the following possibilities exist - Log ? (gt 4.0) - Conjugated ?,? - Unsaturated
ketones, Dienes, Polyenes - Log ? (3.0 4.0) - Aromatic ring (Check IR, NMR)
- Log ? (1.5 2.5) - CO (Check IR)
- Log ? (1.0 1.5) - NO2 (Check IR)
44
Infrared Spectroscopy
- Infrared Spectroscopy References
- Pavia, et al - pp. 873 - 909
- Solomons et al - pp. 79 - 84 821 822
- Infrared Radiation
- That part of the electromagnetic spectrum between
the visible and microwave regions 0.8 ?m
(12,500 cm-1) to 50 ?m (200 cm-1). - Area of Interest in Infrared Spectroscopy
- The Vibrational portion of infrared spectrum
- 2.5 ?m (4,000 cm-1) to 25 ?m (400 cm-1)
- Radiation in the vibrational infrared region is
expressed in units called wavenumbers ( )
45
Infrared Spectroscopy
- Wavenumbers are expressed in units of reciprocal
centimeters (cm-1) i.e. the reciprocal of the
wavelength (?) expressed in centimeters. - ? (cm-1) 1 /
? (cm) - Wave Numbers can be converted to a frequency (?)
by multiplying them by the speed of light (c) in
cm/sec - ? (Hz) ? c c / ? (cm
/sec /cm 1/sec) - Recall E h c / ?
- Thus, wavenumbers are directly proportional to
energy
?
?
46
Infrared Spectroscopy
- Polar Covalent Bonds Dipole Moments
- Organic compounds are organized into families of
compounds on the basis of certain groupings of
atoms, i.e., Functional Groups. - The Electrons between atoms in an organic
compound are shared forming Covalent bonds. - Covalent bonds between atoms with different
electronegativities have an unequal sharing of
the bond electrons setting up an electrostatic
charge difference between the atoms. - The atom with the greater Electronegativity pulls
the electrons closer to it forming a Polar
Covalent Bond.
47
Infrared Spectroscopy
- Polar Covalent Bonds Dipole Moments (Cont)
- The relative strength of the Polar Covalent Bond
impacts the ability of the molecule, i.e., a
Functional Group, to attract or repel other polar
entities (functional groups). - The separation of the positive and negative
charges in a Polar Covalent Bond is referred to
as a Dipole. - A dipole has a Dipole Moment defined as the
product of the magnitude of the partial charges
(in electrostatic units, esu) times the distance
(in cm) of separation. - Only those Covalent bonds with Dipole Moments are
capable of absorbing Infrared Radiation.
48
Infrared Spectroscopy
- The Radiation (Energy) Absorption Process
- The absorption of Infrared Radiation by a Polar
Covalent Bond raises the molecule to a higher
energy state. - This is a Quantized process in which only
selected frequencies are absorbed dependent on
the relative masses of the atoms, the force
constants of the bond (electronegativity), and
the geometry of the atoms. - Covalent Bonds possess Rotational and Vibrational
frequencies. - Every type of bond has a natural frequency of
vibration. - The same bond in different compounds has a
slightly different frequency of vibration.
49
Infrared Spectroscopy
- When the frequencies of Infrared Radiation match
the natural vibrational frequencies of a bond
with a Dipole Moment, the radiation is absorbed
increasing the amplitude of the vibrational
motions of the covalent bonds. - Infrared radiation is absorbed and converted by
organic molecules with polar covalent bonds and
dipole moments into energy of molecular rotation
and molecular vibration. - Rotation - Less than 100 cm-1
(Spectrum is lines) - Vibration - 10,000 cm-1 to 100 cm-1 (Spectrum is
bands) - The vibrational bands appears because each
vibrational energy change is accompanied by a
number of rotational changes - Infrared Spectroscopy is concerned only with the
vibrational spectrum (4,000 cm-1 to 400 cm-1)
50
Infrared Spectroscopy
- Molecular Vibrations
- Absorption of infrared radiation corresponds to
energy changes on the order of 8-40 KJ/mole (2-10
Kcal/mole - The frequencies in this energy range correspond
to the stretching and bending frequencies of the
covalent bonds with dipole moments. - Stretching (requires more energy than bending)
- Symmetrical
- Asymmetrical
- Bending
- Scissoring (in-plane bending)
- Rocking (in-plane bending)
- Wagging (out-of-plane bending)
- Twisting (out of plane bending)
51
Infrared Spectroscopy
- Stretching A rhythmical movement along the bond
axis such that the interatomic distance is
increasing or decreasing. - In any group of three or more atoms at least
two of which are identical - there are two modes
of stretching or bending Symmetric and
Asymmetric - For the Methylene Group (CH2)
H C H
H C H
CH
Symmetric Stretch (2853 cm-1)
Asymmetric Stretch (2926 cm-1)
52
Infrared Spectroscopy
- Bending A change in bond angle between bonds
with a common atom or - A movement of a group of atoms with respect to
the remainder of the molecule
H C H
Scissoring 1450 cm-1 (In Plane)
Wagging 1250 cm-1 (Out of Plane)
H H C
H C H
H H C
Twisting 1250 cm-1 (Out of Plane)
Rocking 750 cm-1 (In Plane)
53
Infrared Spectroscopy
- Thus, no two molecules of different structure
will have exactly the same natural frequency of
vibration, each will have a unique infrared
absorption pattern or spectrum. - Two Uses
- IR can be used to distinguish one compound from
another. - Absorption of IR energy by organic compounds will
occur in a manner characteristic of the relative
strengths of the Polar Covalent Bonds in the
Functional Groups present in the compound thus,
an Infrared Spectrum gives structural information
about the functional groups present in a
molecule. - The absorptions of each type of bond (NH, CH,
OH, CX, CO, CO, CC, CC, CC, CN, etc.) are
regularly found only in certain small portions of
the vibrational infrared region, greatly
enhancing analysis possibilities.
54
Infrared Spectroscopy
- Instrumentation
- Dispersive (Double Beam) IR Spectrophotometer
SplitBeams
Air
Detector
Recorder
Sample
Slit
IR Source
Lenz
Monochromator
The split beams pass into a Monochromator, which
consists of a rapidly rotating sector that passes
each beam to a diffraction grating or prism. The
slowly rotating diffraction grating varies the
wavelength of radiation reaching the
detector. The detector senses the ratio in
intensity between the reference (air) and sample
beams and records the differences on a chart.
55
Infrared Spectroscopy
- Sample Preparation
- Liquid Samples
- 1 to 2 drops of liquid sample are placed between
two single crystals of sodium chloride (Plates) - Note NaCL plates are water soluble keep
dry - Solid Samples soluble in Acetone
- Dissolve sample in acetone
- Evaporate on Salt Plate
- Solid Samples not soluble in acetone
- Make Potassium Bromide (KBR) pellet
- Put plates in plate holder
- Place holder in IR Spectrometer
- Obtain IR Spectrum
- Clean Plates with Acetone
56
Infrared Spectroscopy
- Fourier Transform (FT) Single Beam IR
- Set background (air)
- Press Scan button
- Press Background button
- Verify No. of Scans is 4 if not, push soft key
to set 4 - Press Execute
- Obtain Sample Spectra
- Insert Cell Holder into beam slot
- Press SCAN button
- Select Memory location ( X, Y, or Z)
- Press Execute
57
Infrared Spectroscopy
- Fourier Transform (FT) Single Beam IR (Cont)
- If spectrum bottoms out (might have to check with
instructor), then remove Cell Holder remove top
of Salt Plate wipe lightly with tissue
reassemble and insert cell holder into beam
slot. - Rerun Scan again
- Push Plot to produce chart
- Remove Cell Holder and disassemble
- Clean Salt Plate dry return to instructor
place in desiccator
58
Infrared Spectroscopy
- The Infrared Spectrum
- A plot of absorption intensity ( Transmittance)
on the y-axis vs. frequency on the x-axis. - Transmittance (T) - the ratio of the radiant
power transmitted by a sample to the radiant
power incident on the sample. - Absorbance (A) - the logarithm to base 10 of
the reciprocal of the Transmittance. - A log10 (1 / T)
- Frequency - The x-axis is represented by two
scales - Wavelength (2.5 ? to 25 ? ) (Bottom)
- Wavenumber (4000 cm-1 to 400 cm-1) (Upper)
59
Infrared Spectroscopy
- IR SpectrumKetone
CO CarbonylOvertone
CH2
Methyl Isopropyl Ketone
CH3
Aliphatic C-H Stretch
CO Carbonyl
C5H10O
CAS 563-80-4
60
Infrared Spectroscopy
- IR Spectrum Peak Characteristics
- Primary Examination Regions of the Spectrum
- High Frequency Region - 4000 to 1300 cm-1
- Intermediate (Fingerprint Region) - 1300 to 900
cm-1 - High Frequency Region (Functional Group Region)
- Characteristic Stretching frequencies of such
groups as - C?H, OH, NH, CO, C?O, CN, CC,
CC - The Fingerprint Region - 1300 to 900 cm-1
- Absorption patterns frequently complex
- Bands originate from interacting vibrational
modes - Valuable when used in reference to other regions
- Absorption unique for every molecular species
- Effective use comes from experience
61
Infrared Spectroscopy
- IR Spectrum Peak Characteristics (cont)
- Shape
- Sharp (narrow)
- Broad
- Intensity
- Weak (w)
- Medium (m)
- Strong (s)
- Note Peak intensity is dependent on amount of
sample and sensitivity of instrument therefore,
the actual intensity can vary from spectrum to
spectrum
62
Infrared Spectroscopy
- Principal Frequency Bands
- O-H 3600 cm-1 (Acids, Alcohols)
- N-H 3300 - 3500 cm-1 (Amino)
- (1o - 2 peaks, 2o - 1 peak,
3o 0 peaks) - NO2 1450 1650 cm-1 (2 absorptions)
- CN 2250 cm-1 (Nitrile)
- CC 2150 cm-1 (Acetylene)
- -CC-H 3300 cm-1 (Terminal Acetylene)
- CO 1685 - 1725 cm-1 (Carbonyl)
- CC 1650 cm-1 (Alkene) 2 absorptions
- CC 1450 1600 cm-1 (Aromatic) 4 absorptions
63
Infrared Spectroscopy
- Principal Frequency Bands (Cont)
- CH2 1450 cm-1 (Methylene)
- CH3 1375 1450 cm-1 (Methyl)
- C-O 900 - 1100 cm-1 (Alcohol, Acid, Ester,
Ether, Anhydride) - -C-H Right side of 3000 cm-1 (Saturated Alkane)
- C-H Left side of 3000 cm-1 (Unsaturated
Alkene) - C-H 1667 2000 cm-1 (Aromatic
Overtones) - C-H 2150 cm-1 (Stretch)
64
Infrared Spectroscopy
Functional Type of Frequency Group Vibration
cm-1 Intensity
Alkanes (C-H) (stretch) 3000-2850 s
-CH3 (bend) 1450 1375 m -CH2
(bend) 1465 m Alkenes (CC) (stretch) 3100-3000
m (bend) 1000-650 s Aromatics (stretch) 3150-30
50 s (OOP bend) 1000-650 s Alkyne
(C?) (stretch) 3300 s Aldehyde
(CHO) (stretch) 2900-2800 w (stretch) 2800-2700
w
65
Infrared Spectroscopy
- Correlation Table
- Functional Group Frequency (cm-1) Intensity
- C?C Alkane Not Useful
- CC Alkene 1680-1600 m-w
- Aromatic 1600-1400 m-w
- CC Alkyne 2250-2100 m-w
- CC-H Alkyne (terminal) 3300 s
- CO Anhydride 1810 s
- 1760 s
- Ester 1750-1730 s
- Aldehyde 1740-1720 s
- Ketone (acyclic) 1725-1705 s
- Carboxylic Acid 1725-1700 s
- Amide 1700-1640 s
66
Infrared Spectroscopy
- Correlation Table
- Functional Group Frequency(cm-1)
Intensity - C-O Alcohols, Ethers 1300-1000
s Esters, Acids - O-H Alcohols, Phenols
- Free 3650-3600
m - H-Bonded 3400-3200
m - Carboxylic Acids 3300-2500 m
- N-H Primary Sec Amines 3500
m - CN Nitriles 2260-2240
m - NO Nitro (R-NO2) 1600-1500
s -
1400-1300 s - C-X Fluoride 1400-1000
s - Chloride 800-600
s - Bromide, Iodide lt600
s
67
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 1. Check for the presence of the Carbonyl
group (CO) in the range 1660 1820 cm-1. - If the Carbonyl Group is present, one of the
following types of compounds is present - Carboxylic Acid
- Ester
- Amide
- Anhydride
- Aldehyde
- Ketone
- Acid Chloride
- If the molecule is conjugated (alternating
double single bonds), the strong (CO)
absorption will be shifted to the right by 30
cm-1
68
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 2. Check for the presence of Saturated
Alkane structures - Compounds containing just Methyl (CH3)
Methylene (CH2) groups produce generally simple
IR spectra - CH sp3 absorption is a stretch in the range
3000 2840 cm-1 - Note It is important to remember that the
Alkane sp3 stretch occurs on the
right side of the 3000 cm-1 mark in the IR
spectrum and that Alkene and Aromatic sp2
stretches occur on the left side of the 3000
cm-1 mark (see next slide). - CH3 Methyl groups (CH3) have a characteristic
bending at 1375 cm-1 and a smaller
absorption at 1450 cm-1. - CH2 Methylene groups (CH2) have characteristic
bending at approximately 1465 cm-1
69
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 3. Check for the presence of unsaturated
(CH) sp2 structures. - CH sp2 absorption is a stretch in the range
3000 3100 cm-1, i.e., on the left side of the
3000 cm-1 mark on the x-axis scale. - Step 4. Determine whether the CH bond is
Aliphatic Alkene, Aromatic, or both. - For Alkene CH bonds, look for the CC stretch
at 1600 1650 cm-1, usually an unequal pair of
absorptions. - Out-of-Plan (OOP) bending at 650 1000 cm-1
- Note See next slide or the table on page 895 of
Pavia text for guide to substitution patterns
on substituted alkenes.
70
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Out of Plane (OOP) substitution patterns
(substituted alkenes)
71
Infrared Spectroscopy
- IR SpectrumAliphatic Alkene
1-Hexene
CAS 592-41-6
C6H12
72
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 4 (Cont)
- Aromatic C-H bonds.
- Look for CC stretch - (pair of absorptions at
1450 cm-1 and a pair of absorptions at 1650 cm-1 - Overtone/Combination bands appear between1667
2000 cm-1 - Out-of-Plain (OOP) bending between 650 1000
cm-1 - Note See next slide or the table on page 897 of
Pavia text for guide to substitution
patterns on Benzene ring. - Note The substitution pattern information in
the Overtone area and the OOP
area is duplicative. Use both tables
to confirm substitution pattern
73
Infrared Spectroscopy
74
Infrared Spectroscopy
- IR Spectrum(Aromatic)
Toluene (Methyl Benzene)
C7H8
CAS 108-88-3
75
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 5. Carbonyl Compounds (Carboxylic Acids)
- Strong band of CO group appears in range
1700-1725 cm-1. - Very broad absorption band of the OH group in the
range2400-3400 cm-1. - This broad band will usually obscure the Alkane
C-H stretch bands from 2849-3000 cm-1. - Medium intensity C-O stretch (as in C-OH) occurs
in the range 1210-1320 cm-1
76
Infrared Spectroscopy
- IR SpectrumCarboxylic Acids
Isobutyric Acid
C4H8O2
CAS 79-31-2
77
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 6. Carbonyl Compounds (Esters)
- CO stretch appears in the range 1730-1750 cm-1
- Check for 2 or more C-O stretch bands, one
stronger and broader than the other, in the range
1100-1300 cm-1
78
Infrared Spectroscopy
- IR SpectrumEsters
Methyl Benzoate
C8H8O2
CAS 93-58-3
79
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 7. Carbonyl Compounds (Anhydrides)
- 2 CO stretch bands (1740-1775 cm-1 1800-1830
cm-1) - Conjugation will move these bands to lower
frequency - Multiple C-O stretch bands in the range 900
1300 cm-1
80
Infrared Spectroscopy
- IR SpectrumAnhydrides
Propionic Anhydride
C6H10O3
CAS 123-62-6
81
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 8. Carbonyl Compounds (Amides)
- CO stretch at approximately 1640-1700 cm-1
- N-H stretch (medium absorptions) near 3500 cm-1
- Primary Amino (-NH2) - 2 Peaks (3180 3350 cm-1)
- Secondary Amino (-NH) - 1 Peak (3300 cm-1)
- N-H Scissoring - 1550 - 1640 cm-1
- N-H Bend - 800 cm-1
82
Infrared Spectroscopy
- IR SpectrumAmides
Benzamide
Aromatic Overtones
UnsatdC-H Stretch
-C-N str
N-H Scissoring
NH2 Stretch2 peaks Primary Amino
CC Aromatic
CO Carbonyl
C7H7NO
CAS 55-21-0
83
Infrared Spectroscopy
Acetanilide (N-Phenylacetamide)
- IR SpectrumAmides
C8H9NO
CAS 103-84-4
84
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 9. Carbonyl Compounds (Aldehydes)
- CO stretch appears in the range 1720 - 1740
cm-1 - 2 weak Aldehyde C-H stretch absorptions near 2850
and 2750 cm-1)
85
Infrared Spectroscopy
- IR SpectrumAldehydes
Nonanal
C9H18O
CAS 124-19-6
86
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 10. Carbonyl Compounds (Ketones)
- CO stretch occurs at approximately 1705 1725
cm-1 - Ketones are confirmed when the other five
compound types containing a Carbonyl group have
been eliminated. - Ketone IR Spectra can sometimes be confused with
Ester spectra because of an absorption in the
1100 -1300 cm-1 range similar to the location of
the C-O stretch in esters. Usually, however, the
ester will have 2 or more of the C-O stretch
absorptions.
The Ketone structure produces a medium to strong
absorption in the 1100 1300 cm-1 range due to
coupled Stretching and Bending vibrations
87
Infrared Spectroscopy
- IR SpectrumKetones
Ethyl Isopropyl Ketone (2-Methyl-3-Pentanone)
C6H12O
CAS 565-69-5
88
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 11. Triple Bonds
- Alkynes
- R C C R weak, sharp stretch near 2150 cm-1
- R C C H (Terminal Acetylene)
- Weak, sharp stretch near 2150 cm-1
- and a second stretch at
3300 cm-1 - Nitriles
- C N Medium, sharp stretch near 2250 cm-1
89
Infrared Spectroscopy
Propargyl Alcohol (2-Propyn-1-ol)
IR SpectrumAlkynes (C?C)
CCStretch
Aliphatic C-H Stretch
OH H - Bonded
CH2
C-H Terminal Alkyne Stretch
C-O
C3H4O
CAS 107-19-7
90
Infrared Spectroscopy
IR SpectrumNitriles
Benzonitrile
C7H5N
CAS 100-47-0
91
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 12. - Alcohols Phenols
- Broad absorption near 3600 - 3300 cm-1
- Confirm presence of CO (COH) near 1000 - 1300
cm-1
92
Infrared Spectroscopy
IR SpectrumAlcohols Phenols
2-Naphthol (Nujol Mull)
C10H9O
CAS 135-19-3
93
Infrared Spectroscopy
IR SpectrumAlcohols Phenols
2-Naphthol (CCl4 Soln)
C10H9O
CAS 135-19-3
94
Infrared Spectroscopy
IR SpectrumAlcohols Phenols
2-Naphthol (KBr Disc)
C10H9O
CAS 135-19-3
95
Infrared Spectroscopy
IR SpectrumAlcohols Phenols
2-Butanol
C4H10O
CAS 78-92-2
96
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 13. Ethers
- CO absorptions near 1000 - 1300 cm-1
- Absence of OH
- Absence of CO group
- Aliphatic Ethers give a single strong C-O band
at1120 cm-1 - Unbalanced Ethers will show 2 CO groups
- Phenyl Alkyl Ethers give two (2) strong bands at
about 1040 1250 cm-1
97
Infrared Spectroscopy
IR SpectrumEthers
Butyl Ether(Balanced Ether)
CH3
CH2
Aliphatic C-H Stretch
C-O
CH3(CH2)3 O (CH2)3CH3
C8H18O
CAS 142-96-1
98
Infrared Spectroscopy
IR SpectrumEthers
Phenetole (Unbalanced Phenyl Alkyl Ether)
C8H10O
CAS 103-73-1
99
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 14. Amines
- N-H stretch (Medium absorptions) near 3500 cm-1
- Primary Amino - 2 Peaks
- Secondary Amino - 1 Peak
- Tertiary Amino - No peaks
- N-H Scissoring at 1560 - 1640 cm-1
- N-H Bend at 800 cm-1
100
Infrared Spectroscopy
n-Butylamine(Primary Amine)
IR SpectrumAmines
C4H11N
CAS 109-73-9
101
Infrared Spectroscopy
N-Methylbenzylamine(Sec Amine)
IR SpectrumAmines
AromaticOvertones
N-H Scissoring
Sec-Amino
Unsat C-H Stretch
Sat C-H Stretch
Aromatic ring CC Absorptions
CH3
CH3
-N-H OOP Bending
C-N Str
N-H Scissoring
CH2
OOP Bending Aromatic Monosubstitution
CH2
Aliphatic C-H Stretch
C6H11N
CAS 103-67-3
102
Infrared Spectroscopy
- Analyzing the Spectrum A Suggested Approach
- Step 15. Nitro Compounds
- Two strong absorptions
- Aliphatic Nitro Compounds
- Asymmetric strong stretch 1530 - 1600 cm-1
- Symmetric medium stretch 1300 - 1390 cm-1
- Aromatic Nitro Compounds
- Asymmetric strong stretch 1490 - 1550 cm-1
- Symmetric strong stretch 1315 - 1355 cm-1
103
Infrared Spectroscopy
IR SpectrumNitro Compounds
Nitro Benzene
C6H5NO2
CAS 98-95-3
104
Infrared Spectroscopy
IR SpectrumNitro Compounds
1-Nitro Propane
C3H5NO2
CAS 108-03-2
105
Infrared Spectroscopy
- Step 16. If none of the above apply then the
compound is most likely a - Hydrocarbon
- Alkyl Halide (see slides 105 - 109).
- Hydrocarbons
- Generally, very simple spectrum
- C-H Satd Alkanes 2900 - 3000 cm-1
- Methyl (CH3) 1370 cm-1
- Methylene (CH2) 1450 cm-1
- t-Butyl Group 525 cm-1
- Long Alkane (CH2) Chain 720 cm-1
106
Infrared Spectroscopy
IR Spectrum Alkane
Decane
CH3(CH2)8CH3
C10H22
CAS 124-18-5
107
Infrared Spectroscopy
- Step 17. Halogens
- The Halogens as CH2 - X absorptions show up in
the region (1000 1300 cm-1). - Halogens (Cl, Br, I) show in the Fingerprint
region (485 800 cm-1) as one or two absorptions
see next slide. - Using IR to identify Halogens in this region can
be difficult, especially if OOP Bending
absorptions (used for Substitution Pattern
information) from Alkene and Aromatic unsaturated
Pi (?) bond structures are present. - Halogen identification should be restricted to
Aliphatic Alkane structures containing mainly CH2
CH3 groups. - Iodide and Bromide absorptions in the range 485
650 cm-1 are generally out range on NaCL Salt
Plates, however, if other substrates, e.g.,KBr
pellets, are used, the absorptions are extended
to this range.
108
Infrared Spectroscopy
- Step 17. Halogens (Cont)
- Fluoride 1000 1400 cm-1
- Monofluorides 1000 1200 cm-1
- Polyfluorides 1100 1300 cm-1
- Aryl Fluorides 1100 1250 cm-1
- Chloride (2 or more bands) 540 785 cm-1
- CH2-CL (Bend Wagging) 1230 1300 cm-1
- t-Butyl Group
525 cm-1 - Bromine (KBr Pellets) 510 650 cm-1
- CH2-Br (Bend Wagging) 1190 1250 cm-1
- Aryl Bromides 1030 1075 cm-1
- Iodide (KBr Pellets) 485 600 cm-1
- CH2-I (Bend Wagging) 1150 1200 cm-1
109
Infrared Spectroscopy
IR SpectrumHalogens
2-Bromobutane
Br
CH3
-C-H Satn
CH2
CH2-Br
C4H9Br
CAS 78-76-2
110
Infrared Spectroscopy
IR SpectrumHalogens
1-Chloropropane
C3H7Cl
CAS 540-54-5
111
Infrared Spectroscopy
IR SpectrumHalogens
o-Chlorotoluene
C7H7Cl
CAS 95-49-8
112
Infrared Spectroscopy
IR Spectrum Halogens
T-Pentyl Chloride (2-Chloro-2-MethylButane
T-Pentyl 525 cm-1
CH3
CH2
Saturated Aliphatic C-H Stretch
CH2-Cl
C5H14CL
CAS 594-36-5
113
IR Analysis Scheme
Carbonyl (CO) _at_ 1715-1685 (Conjugation moves
absorption to right 30 cm-1
Yes
No
Acid Ester Amide Anhydride Aldehyde Ketone
Alcohol Amine Ether
Saturation lt 3000 cm-1
Unsaturation gt 3000 cm-1
Alkanes -C-H Methylene -CH2 Methyl -CH3
Alkenes (Vinyl) -CC Alkynes (Acetylenes) -CC Aro
matic -CC
Nitriles
Nitro
Hydrocarbons
114
IR Analysis Scheme
Carbonyl (CO) is Present Acid - Broad OH
Absorption _at_ 3300-2500 cm-1 Ester - C-O
Absorption _at_ 1300-1000 cm-1 Amide - NH Absorption
_at_ 3500 cm-1 (1 or 2 peaks) Anhydride - 2 CO
Absorptions 1810 1760 cm-1 Aldehyde - Aldehyde
C-H Absorptions _at_ 2850 2750 cm-1 Ketone - None
of the above except CO
Carbonyl is Absent Alcohol - Broad OH absorption
_at_ 3300 - 3000 cm-1 Also C-O
absorption _at_ 1300 - 1000 cm-1 Amine - 1 to 2
equal NH absorptions _at_ 3500 cm-1 Ether - C-O
absorption _at_ 1300 - 1000 cm-1
115
IR Analysis Scheme
Saturation
Alkanes -C-H Stretch several absorptions to
right of 3000 cm-1 Methylene -CH2 1450
cm-1 Methyl -CH3 1375 cm-1
Unsaturation
Double Bonds C-H Stretch several absorptions
to left of 3000 cm-1 OOP bending at 1000
650 cm-1 Alkenes (Vinyl) -CC- Stretch (weak) _at_
1675 1600 cm-1
Conjugation moves absorption to
the right Alkynes -CC-H Terminal Acetylene
Stretch at 3300 cm-1 Alkynes (Acetylenes) -CC Str
etch _at_ 2150 cm-1
Conjugation moves absorption to the
right Aromatic (Benzene) C-H Stretch absorptions
also to left of 3000 cm-1 OOP bending at 900
690 cm-1 OOP absorption patterns allow
determination of ring substitution (p.
897 Pavia text) -CC 4 Sharp absorptions (2
pairs) _at_ 1600 1450 cm-1 Overtone absorptions
_at_ 2000 1667 cm-1 Relative shapes and numbers
of peaks permit determination of ring
substitution pattern (p. 897 Pavia text).