SEPARATION OF CHARGED BIOMOLECULES
1 / 3
Title:
SEPARATION OF CHARGED BIOMOLECULES
Description:
The relative mobility is almost a near linear function of the molecular weight. ... bands are observed of lower molecular weight, then we have a multisubunit ... –
Number of Views:229
Avg rating:3.0/5.0
Title: SEPARATION OF CHARGED BIOMOLECULES
1
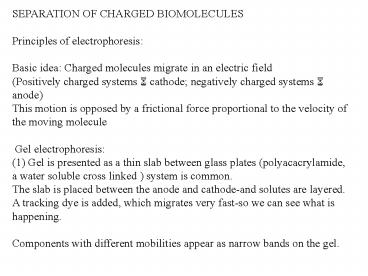
SEPARATION OF CHARGED BIOMOLECULES Principles of
electrophoresis Basic idea Charged molecules
migrate in an electric field (Positively charged
systems 6 cathode negatively charged systems 6
anode) This motion is opposed by a frictional
force proportional to the velocity of the moving
molecule Gel electrophoresis (1) Gel is
presented as a thin slab between glass plates
(polyacacrylamide, a water soluble cross linked )
system is common. The slab is placed between the
anode and cathode-and solutes are layered. A
tracking dye is added, which migrates very
fast-so we can see what is happening. Components
with different mobilities appear as narrow bands
on the gel.
2
Principles of separation by electrophoresis (1)
Important idea- the Ferguson effect The gel
behaves as a molecular sieve so that at higher
concentration of polyacrylamide the mobility is
diminished, and for systems with equal
charge/residue, (DNA for example) this effect is
proportional to the molecular weight or length of
the molecule that is being separated. The
relative mobility is almost a near linear
function of the molecular weight. (2) Carry out
gel electrophoresis in the presence of sodium
dodecyl sulphate (SDS). Under these conditions ,
the secondary tertiary and quaternary structure
of the protein is borken down. The-charges on the
protein make its own charge irrelevant. The
unfolded chain has a length and a (-) charge
which are each proportional to the length of the
chain.These molecules have relative mobilities
which depend only on their lengths and therefore
separation is based only on masses.
3
Example Protein with
subunits Carry out SDS gel electrophoresis
experiments in the presence of ß-mercaptoethanol
(HSCH2CH2OH) , which breaks S-S bonds and in the
absence of this molecule. If you find one band of
the same MW under each of these conditions, it is
safe to conclude that the protein exists as a
single polypeptide chain. If bands are observed
of lower molecular weight, then we have a
multisubunit structure. Isoelectric focusing
gels At the isoelectric point of a protein,
the net charge is zero and the protein will not
migrate in an E field. If we can create a stable
pH gradient across a gel. Each protein will
migrate to the position of its isoelectric point
and remain there. Separation is based therefore
on charge.