Chapter 14: Solutions - PowerPoint PPT Presentation
1 / 64
Title: Chapter 14: Solutions
1
Chapter 14 Solutions
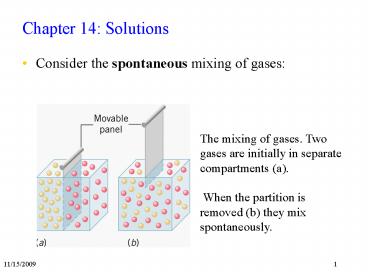
- Consider the spontaneous mixing of gases
The mixing of gases. Two gases are initially in
separate compartments (a). When the partition
is removed (b) they mix spontaneously.
2
The Process Of Dissolution
- Polar solutes interact with and dissolve in polar
solvents - Non-polar solutes interact with and dissolve in
non-polar solvents
Dipole- dipole interaction (H-bonding)
London forces
3
Miscibility of Liquids
- Liquids that can dissolve in one another are
miscible, while insoluble liquids are immiscible - Ethanol and water are miscible, while benzene and
water are not
4
Learning Check
- Which of the following are miscible in water?
water
ammonia
carbon disulfide
acetic acid
5
- This can be summarized as the rule of thumb like
dissolves like - The basic principles remain the same when the
solutes are solids - Sodium chloride dissolves when it is added to
water - The sodium and chloride ions are hydrated or
surrounded by water molecules - The general term for surrounding a solute
particle by solvent molecules is solvation
6
Dissolution Of An Ionic Compound In Water
- Positive end of the dipole of the water surrounds
the anions of the ionic solid, extracting them
from the lattice - Negative end of the dipole orients toward the
cations, surrounding and extracting them from the
lattice
7
Dissolution Of A Polar Compound In Water
- Dipole of the water interacts with the oppositely
charged dipoles of the solid, extracting them
from the crystal
8
Enthalpy (Heat) Of Solution
- Heat of solution (??soln ) is the energy
exchanged when a solute dissolves in a solvent at
constant pressure - Enthalpy is a state function, so the pathway can
be written in any way and the result will be the
same - When ??soln0, solution is called an ideal
solution
9
Dissolution Of An Ionic Solid
- Visualized in steps
- step1 ionic solid breaks apart into vapor phase
lattice energy (U) - step 2 vapor phase interacts with solvent
solvation energy (?Hsolv) if solvent is water,
(??hydration)
??soln (ion in water) U ??solvation
10
- Consider the formation of aqueous potassium
iodide - The lattice energy of KI is 632 kJ mol 1
- The hydrations energy of KI is 619 kJ mol1
- Total 13 kJ mol1 (the value from experiment is
20.33 kJ mol1) - The formation of this solution of aqueous
potassium iodide is endothermic
11
Dissolution Of KI In Water
12
Dissolution Liquid In Liquid
- Step1 solute expands
- Step2 solvent expands
- Step 3 solute solvent mix
- If the ??soln0, we have an ideal solution
??soln ??1 ??2 ??3
13
Dissolution Liquid in Liquid (Non-Ideal)
14
Dissolution Gas In Liquid
- Only very weak attractions exist between gas
molecules (there are no attractions in ideal
gases) - Thus, when making a solution with a gas solute
the energy required to expand the solute is
negligible
- step 1 expansion of solvent
- step 2 mixing
- ??soln ??1 ??2
15
- Solubility is the mass of solute that forms a
saturated solution with a given mass of solvent
at a specified temperature - The units are typically grams of solute per 100 g
of the solvent - If extra solute is added to a saturated solution,
the extra solute will remain as a separate phase - A dynamic equilibrium exists between the solute
in the two phases
16
Saturated Solutions
- Solute is at equilibrium with the dissolved
solute - Addition of more dissolved solute results in
supersaturation and precipitation of excess solid - The presence of less solute than the solubility
results in an unsaturated solution
17
Solubility Varies With Temperature
- Solubility may increase or decrease with
increasing temperature - The extent to which temperature has an effect is
specific to the solute and solvent - Most gases are less soluble in water at high
temperature, while most solids are more soluble
18
- The solubility of gases in water usually
decreases with temperature (see Table 11.2, page
611), for example
19
Case Study Dead Zones
- During the industrial revolution, factories were
built on rivers so that the river water could be
used as a coolant for the machinery. The hot
water was dumped back into the river and cool
water recirculated. After some time, the rivers
began to darken and many fish died. The water
was not found to be contaminated by the
machinery. What was the cause of the mysterious
fish kills?
increased temperature lowered amounts of
dissolved oxygen
20
How an increase in pressure increases the
solubility of a gas in a liquid. (a) At some
specific pressure, an equilibrium exists between
the vapor phase and the solution. (b) An increase
in pressure puts stress on the equilibrium. More
gas molecules dissolve than are leaving the
solution. (c) More gas has dissolved and
equilibrium has been restored.
21
- The gases in air are not very soluble in water
under ordinary pressure - The solubility increases as the pressure is
increased
The amount of gas that dissolves in water
increases as the pressure is raised.
22
Pressure Effects On Solubility Of Gases
- CgaskHPgas
- C concentration of dissolved gas (M)
- kH Henrys Constant
- P pressure applied to system (mm Hg)
- kH (M/mm Hg)
- N2 8.4210 -7
- O2 1.6610-4
- CO2 4.4810-5
- Gases are all more soluble at higher pressures
(the cause of the bends)
example constants
23
Learning Check
- What is the concentration of dissolved nitrogen
in a solution that is saturated in N2 at 2.0 atm
kH 8.4210 -7 (M / mm Hg)
- CgkHPg
- Cg 8.4210 -7 (M / mm Hg) 760 mm Hg/1 atm
2.0 atm - Cg1.3 10-3 M
24
Case Study
- When you open a bottle of seltzer, it fizzes.
How should you store it to increase the time
before it goes flat?
Gases are more soluble at low temperature and
high pressure. Cap it and cool it.
25
- Gas molecules with polar bonds are much more
soluble in water than nonpolar molecules like
oxygen and nitrogen - Some gases have increased solubility because they
react with water - For example
26
Units of Concentration
- Molar concentration or molarity, mol/L, is
convenient for the stoichiometry of chemical
reactions in solutions - Two other temperature-intensive concentrations
are common - Percent Concentrations
- Also called percent by mass or percent by weight
- This is sometimes indicated (w/w) where w
stands for weight - The (w/w) is often omitted
27
- Concentrations are sometimes reported as percent
by mass/volume of (w/v) - Percentages are parts per hundred (pph)
- Other concentrations include parts per million
(ppm) and parts per billion (ppb) - 1 ppm 1 g component in 106 g mixture
- 1 ppb 1 g component in 109 g mixture
28
- The number of moles of solute per kilogram
solvent is called the molal concentration or
molality (m) - Dont confuse molality and molarity
29
Colligative properties (vapor pressure, BP
elevation, osmotic pressure)
- Colligative properties depend mostly on the
relative populations of particles in mixtures,
not on their chemical identities - Solutes that cant evaporate from solution are
called nonvolatile solutes - All solutions of nonvolatile solutes have lower
vapor pressures than their pure solvents
30
Raoults law plot. When the vapor pressure of a
solution is plotted against the mole fraction of
solvent, the result is a straight line.
(a) With a high number of solvent molecules, the
rate of evaporation and condensation is
relatively high. (b) When some of the solvent is
replaced by a nonvolatile solute, the rate of
evaporation and the vapor pressure decrease.
31
- For dilute solution, Raoults law applies
- The change in vapor pressure can be expressed as
32
- When a solution is made from two components that
can evaporate, the vapor contains molecules of
each component - Each component is described by Raoults law,
using the labels A and B
33
- For an ideal, two-component solution of volatile
components
The vapor pressure of an ideal, two-component
solution of volatile components (A and B).
34
Learning Check
- The vapor pressure of 2-methylheptane is 233.95
torr at 55C. 3-ethylpentane has a vapor
pressure of 207.68 at the same temperature. What
would be the pressure of the mixture of 78.0g
2-methylheptane and 15 g 3-ethylpentane?
- PsolutionXAP0AXBP0B
- mole 2-methylheptane 78.0g/114.23 g/mol
0.68283 mol - mole 3-ethylpentane 15g/100.2 g/mol 0.1497 mol
- X2-methylheptane0.82737
P 230 torr
35
Learning Check
- The vapor pressure of 2-methyl hexane is 37.986
torr at 15C. What would be the pressure of the
mixture of 78.0g 2-methylhexane and 15 g
naphthalene which is nearly non-volatile at this
temperature?
- PsolutionXsolventP0solvent
- mol 2-methylhexane 78.0g/100.2 g/mol 0.778443
mol - mol naphthalene 15 g/128.17 g/mol 0.11703
- X2-methylhexane 0.869309
- Psolution 0.869309 37.986 torr
- P33.02 torr
36
- Solutes affect the boiling and freezing point of
solutions (relative to the pure solvent)
Phase diagrams for water and an aqueous solution
(not to scale). (a) Phase diagram for pure water.
(b) Phase diagram for an aqueous solution of a
nonvolatile solute.
37
- The increase in boiling point is called the
boiling point elevation - The decrease in freezing point is called the
freezing point depression - Simple expressions relate the molality (m) to the
temperature change
38
Some BP/FP Constants
39
Learning Check
- According to the Sierra Antifreeze literature,
the freezing point of a 40/60 solution of sierra
antifreeze and water is -4F. What is the
molality of the solution?
-4F 1.8 (C) 32 -20. C
11m
40
Learning Check
- In the previous sample of a Sierra antifreeze
mixture, 100 mL is known to contain 42 g of the
antifreeze and 60. g of water, what is the molar
mass of the compound found in this antifreeze if
it has a freezing point of -4F?
0.66 mol solute
64 g/mol solute
41
Learning Check
- In the previous sample of a Sierra antifreeze
mixture, the freezing point is -4F? What will
be its boiling point?
from before -4F 1.8 (C) 32 -20. C
T105 C
42
Ionic Solutes Affect Colligative Properties
Differently Than Non-ionic Solutes
- substances that ionize make more particles in a
solution than their own concentration suggests - i is a factor that demonstrates how many ions
are formed per formula unit or molecule - the apparent molality of particles is then im.
NaCl(aq) ltgt Na Cl-
ex. 1.0 m NaCl gt 2.0 m
H3COOH(aq) ltgt H3COO- H
43
Learning Check
- In preparing pasta, 2 L of water at 25C are
combined with about 15 g salt (NaCl, MM
58.44g/mol) and the solution brought to a boil.
What is the expected boiling point of the water?
?TimKbp
mass of water volume density 2000 mL 1.0
g/mL 2000g water 2 kg
m0.25667 mol / 2kg 0.123
mol NaCl 15g / 58.44 g/mol mol NaCl 0.25667
T100.1 C
44
Case Study
- Suppose you run out of salt. What mass of sugar
(C12H22O11, MM342.30 g/mol) added to 2 L of
water would raise the temperature of water by
0.10 C?
?TimKbp
mass of water volume density 2000 mL 1.0
g/mL 2000g water 2 kg
0.196 m? mol / 2kg 0.39215mol
m.196
0.39215 mol ?g / 342.30 g/mol mass sucrose 130
g
45
Examples
- Beer is 6.25 by weight ethanol in water. Its
density is about 1.01 g/mL. What is its expected
boiling point?
?Tm Kbp
100.74CTbp
46
Units Of Concentration (Recap)
- Molarity (M) moles solute / L solution
- changes with Temperature
- Molality (m) moles solute/kg solvent
- mole fraction (X)
- X moles component/ total moles
- Percent by mass ()
- (mass solute / mass solution)100
47
Units Of Very Low Concentrations
- Parts per million (ppm)
- µg solute/mL soln
- Parts per billion (ppb)
- ng solute/ mL soln
- for extremely dilute solutions mostly solvent is
present - When the solvent is water (d1g/mL) thus for ppm
( µg solute/g soln) a 1/106 magnitude difference
is given, leading to the name 1 part per 1
billion
48
Raoults Law
- Vapor pressure of a liquid varies as a function
of purity - X mole fraction of solvent
- P0 vapor pressure of pure solvent
- PsolutionXsolventP0solvent
- PsolutionXAP0AXBPB0
- Where A and B are both volatile components.
49
Solute Effects On Phase Changes
- Regardless of the identity of the dissolved
particles, the presence of an impurity will
result in a change in the boiling point and
freezing point. - The effect is solely dependent on the nature of
the solvent, a factor labeled K, and the
concentration of particles present (molality) - ?TmK
- boiling point elevation ?TTmix-Tpure
- freezing Point Depression ?TTpure-Tmix
50
- In living things, membranes of various kinds keep
mixtures organized and separated - These membranes are called semipermeable because
they are selective as to what can pass through
them - The process of letting water and small molecules
through a membrane is called dialysis, and the
membrane is called a dialyzing membrane
51
- An osmotic membrane is a semipermeable membrane
that lets only solvent molecules through - The net shift of solvent molecules (usually
water) through an osmotic membrane is called
osmosis - During osmosis, the solvent flows from the less
concentrated side to the more concentrated side - The flow of solvent molecules increases the
concentration of solute on the less concentrated
side and decreases the concentration of solute on
the more concentrated side of the osmotic membrane
52
Osmosis and osmotic pressure. (a) Before osmosis.
(b) Net flow of solvent into the tube osmosis
has occurred. (c) The back pressure required to
prevent osmosis is called the osmotic pressure.
53
(0.821 L atm mol-1 K-1)
54
- Colligative properties depend on the
concentration of particles - Strong electrolytes, like NaCl, should produce
(nearly) two moles of solute particles for mole
of NaCl that dissolves - The vant Hoff factor i scales the solute
molatity to the correct number of particles
55
- The vant Hoff factor is equivalent to a percent
ionization - In general, it varies with concentration (see
Table 14.4, page 632)
56
- Solute particles must be small to form
homogeneous solutions - Larger particles can form a suspension
- To form a suspension the (solute) particles must
be larger than about 1000 nm in one dimension - A colloidal dispersion or colloid is a mixture
in which the dispersed particles have at least
one dimension in the range 1 to 1000 nm
57
Learning Check Osmosis
- A solution of D5W, 5 dextrose (C6H1206) in water
is placed into the osmometer shown at right. It
has a density of 1.0 g/mL. The surroundings are
filled with distilled water. What is the
expected osmotic pressure at 25C?
58
Learning Check
- For a typical blood plasma, the osmotic pressure
at body temperature (37C) is 5409 mm Hg. If the
dominant solute is serum protein, what is the
concentration of serum protein?
59
Relative Concentration Terms In Osmosis
- Hypotonic solutions have lower ion concentrations
than the cells. - Hypertonic solutions have higher ion
concentrations than the cells - Isotonic solutions have the same ion
concentration as the cells
60
(No Transcript)
61
Dialysis
- Pores on the semi-permeable membrane may be of
varied size - In dialysis, the pores are fairly large, allowing
transfer of solvent, ions, and small proteins - Larger cells, such as red blood cells are
prevented from passing through the pores - The dialysis bath may be enriched in substances
lacking in the blood, and is hypotonic in waste
products in the blood - Exchange of vital components may be made
62
False Solutions
- Suspensions-solids of sufficient size (10µm)
float in a solvent may settle, be centrifuged,
or be filtered - Colloids-particles (1-1000 nm) have slight static
charge from interaction and move via Brownian
motion
63
How Soaps And Detergents Work
- Surfactants are molecules with both polar and a
non-polar ends - Hydrophobic (non-polar) end embeds into non-polar
solutes and the hydrophilic end is exposed on the
outside - Microencapsulated solute called a micelle can now
be carried away by the water - This is how soaps act to dissolve non-polar fats
64
Osmosis (Recap)
- When a solution, surrounded by a semi-permeable
membrane, is placed into another solution,
solvent molecules flow from areas of low
concentration to areas of high concentration - As this occurs, the height of liquid rises in the
higher concentration solution, building up
Osmotic pressure (p) - pMRT
- the concentration, is in molarity, M
- TTemperature, in Kelvin
- RIdeal Gas Constant, 0.082057 Latm/molK
- The basis for kidney function, rising sap, and
dialysis