CHIMIE DOUCE: SOFT CHEMISTRY - PowerPoint PPT Presentation
1 / 46
Title:
CHIMIE DOUCE: SOFT CHEMISTRY
Description:
... (CdSe)n + n/2C2H6 + nnBu3P Addition of reagent nucleation aggregation capping and stabilization nMe2Cd + nnBu3PSe + mnOct3PO (nOct3PO)m(CdSe) ... – PowerPoint PPT presentation
Number of Views:66
Avg rating:3.0/5.0
Title: CHIMIE DOUCE: SOFT CHEMISTRY
1
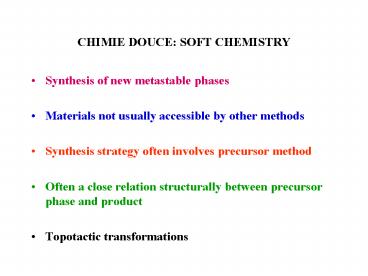
CHIMIE DOUCE SOFT CHEMISTRY
- Synthesis of new metastable phases
- Materials not usually accessible by other methods
- Synthesis strategy often involves precursor
method - Often a close relation structurally between
precursor phase and product - Topotactic transformations
2
CHIMIE DOUCE SOFT CHEMISTRY
- Tournaux synthesis of a new form of TiO2
- Beyond Rutile, Anatase, Brookite and Glassy
form!!! - KNO3 (ToC) ? K2O (source)
- K2O 4TiO2 (rutile, 1000oC) ? K2Ti4O9
- K2Ti4O9 HNO3 (RT) ? H2Ti4O9.H2O
- H2Ti4O9.H2O (500oC) ? 4TiO2 (new slab structure)
2H2O
3
KIRKENDALL EFFECT IN TOURNAUX SYNTHESIS OF SLAB
FORM OF TiO2
- 16K - 4Ti4 36TiO2 ? 8K2Ti4O9
- 4Ti4 - 16K 9K2O ? K2Ti4O9
- Overall reaction stoichiometry
- 9K2O 36TiO2 ? 9K2Ti4O9
- RHS/LHS 8/1 Kirkendall Ratio
4
RUTILE CRYSTAL STRUCTURE
5
SEEING THE 1-D CHANELS IN RUTILE
6
NEW METASTABLE POLYMORPH OF TiO2 BASED ON K2Ti4O9
SLAB STRUCTURE - (010) PROJECTION SHOWN
1
Topotactic loss of H2O from H2Ti4O9 to give
Ti4O8 (TiO2 slabs) plus H2O, where two bridging
oxygens in slab are protonated (TiOHTiOTiOH)
1
1/2
1/2 x2
1
1/3 x2
1
1/3
1/3 x2
1/2
1/3
1/3 x2
1/3
K at y 3/4
1/2
K at y 1/4
Different to rutile, anatase or brookite forms of
TiO2
7
CHIMIE DOUCE SOFT CHEMISTRY
- Figlarz synthesis of new WO3
- WO3 (cubic form) 2NaOH ? Na2WO4 H2O
- Na2WO4 HCl (aq) ? gel
- Gel (hydrothermal) ? 3WO3.H2O
- 3WO3.H2O (air, 420oC) ? WO3 (hexagonal tunnel
structural form of tungsten trioxide) - More open tunnel form than cubic ReO3 form of WO3
8
Slightly tilted cubic polymorph of WO3 with
corner sharing Oh WO6 building blocks, only
protons and smaller alkali cations can be
injected into cubic shaped voids in structure to
form bronzes like NaxWO3 and HxWO3
1-D hexagonal tunnel polymorph of WO3 with corner
sharing Oh WO6 building blocks, can inject larger
alkali and alkaline earth cations into structure
to form bronzes like RbxWO3 and BaxWO3 as well as
HxWO3 a 1D proton conductor having mobile protons
diffusing from O site to site along channels
9
Apex sharing WO6 Oh building blocks
Hexagonal tunnels
Injection of larger M cations like K and Ba2
than maximum of Li and H in c-WO3
Structure of h-WO3 showing large 1-D tunnels
10
Functional device, LED, laser, sensor, biolabel
Growth and ligand capping of nanocluster core
Ligand capping arrested growth of nanocluster
core
High T solvent, ligand, protection, amphiphilic
amines, carboxylic acids, phosphines, phosphine
oxides, phosphonic acids
Inorganic precursor, oxides, sulphides, metals,
nucleation of nanocluster seed
11
Arrested nucleation and growth synthetic method
for making semiconductor nanoclusters in a
high-boiling solvent. Adding a non-solvent
causes the larger nanocrystals to precipitate
first, allowing size-selective precipitation and
nanocluster scaling laws to be defined
nMe2Cd nnBu3PSe mnOct3PO ? (nOct3PO)m(CdSe)n
n/2C2H6 nnBu3P
12
ARRESTED GROWTH OF MONODISPERSED NANOCLUSTERS
- Hydrophobic sheath of alkane chains of surfactant
make the nanoclusters soluble in non-polar
solvents - crucial for achieving purification and
size selective crystallization of the
nanoclusters. - nMe2Cd nnBu3PSe mnOct3PO ? (nOct3PO)m(CdSe)n
n/2C2H6 nnBu3P - Tributylphosphine selenide in a syringe is
rapidly injected into a 300?C solution of
dimethyl cadmium in trioctylphosphine oxide
surfactant-ligand-solvent, known as TOPO.
13
SIZE SELECTIVE CRYSTALLIZATION OF LIGAND CAPPED
NANOCLUSTERS
- Gradually add non-solvent acetone to a toluene
solution of capped nanoclusters - Causes larger crystals to precipitate then
smaller and smaller crystals as the non-solvent
concentration increases. - Smaller ones more soluble because of easier
solvation of less dense packed alkanethiolate
chains.
14
SIZE SELECTIVE CRYSTALLIZATION OF LIGAND CAPPED
NANOCLUSTERS
- When non-solvent added, nc-nc contacts become
more favorable than nc-solvent interactions. - Larger diameter capped nanoclusters interact via
the chains of the alkanethiolate capping ligands
more strongly than the smaller ones due to the
smaller curvature of their surface and the
resulting greater interaction area. - As a result they are caused to flocculate that
is aggregate and crystallize first.
15
SIZE SELECTIVE CRYSTALLIZATION OF LIGAND CAPPED
NANOCLUSTERS
- Process repeated to obtain next lower size
nanoclusters and procedure repeated to obtain
monodispersed alkanethiolate capped gold
nanoclusters. - Further narrowing of nanocluster size
distribution achieved by gel electrophoresis an
electric field driven size exclusion separation
stationary phase.
16
BASICS OF NANOCLUSTER NUCLEATION, GROWTH,
CRYSTALLIZATION AND CAPPING STABILIZATION
?Gb gt ?Gs
supersaturation
nucleation
aggregation
capping and stabilization
nMe2Cd nnBu3PSe mnOct3PO ? (nOct3PO)m(CdSe)n
n/2C2H6 nnBu3P
17
EgC EgB (h2/8R2)(1/me 1/mh) - 1.8e2/?R
Coulomb interaction between e-h
Quantum localization term
CAPPED MONODISPERSED SEMICONDUCTOR NANOCLUSTERS
nMe2Cd nnBu3PSe mnOct3PO ? (nOct3PO)m(CdSe)n
n/2C2H6 nnBu3P
18
(No Transcript)
19
(No Transcript)
20
SIZE DEPENDENT OPTICAL ABSORPTION SPECTRA OF
CAPPED CDSE NANOCLUSTERS, SYNTHESIS AND
CHARACTERIZATION OF NEARLY MONODISPERSE CdE (E
S, Se, Te) SEMICONDUCTOR NANOCRYSTALLITES, MURRAY
CB, NORRIS DJ, BAWENDI MG, JOURNAL OF THE
AMERICAN CHEMICAL SOCIETY 115 (19) 8706-8715 SEP
22 1993)
21
SIZE AND COMPOSITION DEPENDENCE OF THE OPTICAL
EMISSION SPECTRA OF CAPPED InAs (RED), InP
(GREEN) AND CdSe (BLUE), BRUCHEZ, M.JR MORONNE,
M. GIN, P. WEISS, S. ALIVISATOS, A.P.
SEMICONDUCTOR NANOCRYSTALS AS FLUORESCENT
BIOLOGICAL LABELS, SCIENCE 1998, 281, 2013
22
PXRD, MALDI-MS, TEM CHARACTERIZATION OF CLUSTER
CORE, CLUSTER SEPARATION LIGAND SHEATH,
Nanocluster Synthetic Control size, shape,
composition, surface chemical and physical
properties, separation, amorphous, crystalline
Do it yourself quantum mechanics synthetic
design of optical, electrical, magnetic properties
23
ARRESTED GROWTH OF MONODISPERSED NANOCLUSTERS
CRYSTALS, FILMS AND LITHOGRAPHIC PATTERNS
nMe2Cd nnBu3PSe mnOct3PO ? (nOct3PO)m(CdSe)n
n/2C2H6 nnBu3P
24
MONODISPERSED CAPPED CLUSTER SINGLE CRYSTALS
Rogach AFM 2002
methanol
2-propanol
toluene
25
TRI-LAYER SOLVENT DIFFUSION CRYSTALLIZATION OF
CAPPED NANOCLUSTER SINGLE CRYSTALS. MeOH TOP
LAYER, TOLUENE BOTTOM LAYER, 2-PROPANOL MIDDLE
BUFFER LAYER - OMITTING THE BUFFER LAYER CREATED
ILL-DEFINED CRYSTALS, A NEW APPROACH TO
CRYSTALLIZATION OF CdSe NANOPARTICLES INTO
ORDERED THREE-DIMENSIONAL SUPERLATTICES, TALAPIN
DV, SHEVCHENKO EV, KORNOWSKI A, GAPONIK N, HAASE
M, ROGACH AL, WELLER H, ADVANCED MATERIALS, 13
(24) 1868, 2001
26
GOLD ATOMIC DISCRETE STATES
GOLD CLUSTER DISCRETE MOLECULE STATES
GOLD QUANTUM DOT CARRIER SPATIAL AND QUANTUM
CONFINEMENT
GOLD COLLOIDAL PARTICLE SURFACE PLASMON 1850
MICHAEL FARADAY ROYAL INSTITUTION GB PIONEER OF
NANO!!!
BULK GOLD PLASMON
27
SELF-ASSEMBLING AUROTHIOL CLUSTERS
Diagnostic cluster size dependent optical plasmon
resonance originating from dipole oscillations of
conduction electrons spatially confined in
nanocluster wavelength plasmon depends on size,
type of capping ligand and nature of the
environment of nanocluster also size dependent
electrical conductivity hopping from cluster to
cluster - useful in nanoelectronic devices and
nanooptical sensors Faraday would be pleased!!!
HAuCl4(aq) Oct4NBr (Et2O) ? Oct4NAuCl4
(Et2O) nOct4NAuCl4(Et2O) mRSH (tol) 3nNaBH4
? Aun(SR)m (tol)
28
Relationship between alkanethiolate polymer,
nanocluster and self-assembled monolayer
29
SIZE SELECTIVE CRYSTALLIZATION OF SELF-ASSEMBLING
AUROTHIOL CLUSTERS Aun(SR)m
- Gradually adding a non-solvent such as acetone
to a toluene solution of capped gold nanoclusters
first causes larger crystals to precipitate, then
smaller and smaller crystals, as the non-solvent
concentration increases. Smaller ones more
soluble because of easier solvation of less dense
packed alkanethiolate chains. - When non-solvent added, nc-nc contacts become
more favorable than nc-solvent interactions.
Larger diameter capped gold nanoclusters interact
via the chains of the alkanethiolate capping
ligands more strongly than the smaller ones due
to the smaller curvature of their surface and the
resulting greater interaction area. As a result
they are caused to flocculate that is aggregate
and crystallize first. - Process repeated to obtain next lower size
nanoclusters and procedure repeated to obtain
monodispersed alkanethiolate capped gold
nanoclusters.
30
CAPPED METAL CLUSTER CRYSTAL
CLUSTER SELF-ASSEMBLY DRIVEN BY HYDROPHOBIC
INTERACTIONS BETWEEN ALKANE TAILS OF
ALKANETHIOLATE CAPPING GROUPS ON GOLD
NANOCRYSTALLITES
U.Landman AM 1996
31
Plasmonics Basics Size Effects
32
Plasmonics Basics Size Effects
- What is the the surface plasmon resonance of gold
nanostructures. On the top left corner is shown
how the electron cloud of free-electrons in the
gold respond to an oscillating electromagnetic
field, depending on the shape and orientation of
the particle. The formation of a dipole causes
the emergence of a resonance at a specific
wavelength, as shown on the right by the
representative absorbance spectra. In the case of
spherical particles the plasmon resonance occur
at a single frequency, while for elongated
nanocrystals you can have two resonance
frequencies related with the two dipole
oscillation modes (longitudinal or transverse). - In the bottom part of the Figure is shown the
origin of the absorbance features according to
the Mie theory. The absorbance A is expressed as
the product of two terms. The first term is
scattering-related and has a 1/l dependence,
while the second term is exclusively dependent on
the dielectric constants of the metal and the
surrounding medium. This last term represent the
resonant plasmon mode which is shown as a peak
centered at the surface plasmon resonance
wavelength lSPR. The product of the two terms is
the spectrum observed experimentally.
33
SURFACE PLASMON RESONANCE MIE THEORY
- Extinction coefficient from Mie theory is the
exact solution to Maxwells electromagnetic field
equations for a plane wave interacting with a
homogenous sphere of radius R with the same
dielectric constant as bulk metal (scattering and
absorption contributions). - em is the dielectric constant of the
surrounding medium sensitive to environment - e e1 ie2 is the complex dielectric constant
of the particle - Resonance peak occurs whenever the condition e1
-2em is satisfied sensitive to change in em of
environment hence use as a surface plasmon sensor - This is the SPR peak which accounts for the
brilliant colors of various metal nanoparticles
form factors can be introduced to account for
non-spherical shape Gans modification of Mie
theory.
34
Extinction spectra calculated using Mie theory
for gold nanospheres with diameters varying from
5 nm to 100 nm.
35
Detecting Biomolecules with Gold
NanocrystalsSelf Assembly and Plasmon Coupling
36
Detecting Biomolecules with Gold
NanocrystalsSelf Assembly and Plasmon Coupling
- The coupling of plasmons can be used for the
detection of oligonucleotides in solution. Gold
nanocrystals can be produced with
thiol-functionalized oligonucleotides bound to
their surface a construct which we call the
probe. The oligonucleotides on the nanocrystals
are synthesized to be complementary to the ones
one wants to detect. The ultraspecific binding of
oligonucleotides for their complementary strand
allows the particles to bind very efficiently to
the analytes in solution. Such binding of two
nanocrystals to the same analyte brings the
nanocrystals very close together thus enabling
the coupling of the plasmons. - As shown in the diagram below, once the
nanocrystals are close the dipole can extend over
the ensemble of the two nanocrystals (as in
resonance r2) while for single isolated particle
the dipole is confined to the particle itself
(resonance r1). The simultaneous presence of r1
and r2 resonances leads to an effective red shift
of the absorbance peak of the nanocrystals thus
changing their color, as shown in the photos
thereby enabling detection of a specific
oligonucleotide which shows complementary
Watson-Crick base pairing.
37
Gold Nanocrystals to Gold Nanorods
- Gold nanocrystal ncAu seed mediated growth of
gold nanorods nrAu - ncAu seeds obtained by aqueous sodium borohydride
reduction of HAuCl4 with sodium citrate surface
stabilization - nrAu obtained by surfactant (trimethylcetylammoni
um bromide CTAB) directed re-growth of ncAu seeds
using ascorbic acid mild reducing agent of HAuCl4
38
Non-Spherical Shapes -Gans Modified Mie Theory
39
Au Nanorods Shape Selective AdditivesAspect
Ratio Tunes Longitudinal NOT Transverse SPR Modes
(a) L 46 nm, w 22 nm (b) L 61 nm, w 22
nm (c) L 73 nm, w 22 nm (d) L 75 nm, w
22 nm (e) L 89 nm, w 22 nm (f) L 108 nm,
w 22 nm. The right panel shows a representative
TEM image of the sample corresponding to
spectrum-f.
Calculated Gans Theory Gold Nanorod w 20 nm
40
Gold NanorodsAspect Ratio Tunes Longitudinal
NOT Transverse SPR Modes
NANOCHEMISTRY CURES CANCER CANCER CELL TARGETED
GOLD NANOROD ATTACHMENT BURN AWAY THOSE NASTY
CANCER CELLS BY NANORODS ABSORBING NIR PLASMON
AND TRANSFERING HEAT TO CANCER CELL
PHOTOTHERMAL CANCER THERAPY
41
Nano Medicine - Photothermal Cancer Therapy Using
Gold Nanorods
42
Nano MedicinePhotothermal Cancer Therapy Using
Gold Nanorods
- In the top part of the Figure you can see how the
plasmons relax back to the equilibrium state
after being excited at their resonance. The
relaxation occurs through emission of heat that
can be used for killing cells to which they are
selectively attached. - In the middle part of the Figure is shown from
the left the absorption/scattering spectrum of
the biological tissues and water the windows of
low absorbance are indicated by the pink areas.
In the next spectrum is shown how the different
absorbances of the tissues at different
wavelengths affect the intensity of light
propagating in them as it is shown in the graph
the light with wavelength 1000 nm will propagate
further than light 500 nm, which is instead
strongly absorbed. On the right is a
representative absorbance spectrum of gold
nanorods highlighting how the second resonance
peak can be made to fit in the biological window,
thus increasing its potential for photothermal
therapy. - In the bottom part of the Figure are shown three
different lines of cells (nonmalignant HaCat,
malignant HSC and malignant HOC cells) after
having exposed them to a strong NIR laser light
(the circle highlights the area of exposure). The
gold nanorods were made to bind selectively to
the malignant cells thanks to an active targeting
protocol. As you can see the nonmalignant cells
were not harmed since they were not targeted by
the nanorods. The malignant cells instead
suffered strong damage because they were targeted
by the nanorods and because the nanorods heated
up upon irradiation with the laser.
43
(No Transcript)
44
CAPPED FePt FERROMAGNETIC NANOCLUSTER
SUPERLATTICE HIGH-DENSITY DATA STORAGE MATERIALS
45
NANOMAGNETIC SEPARATIONS OF BIOLOGICAL MOLECULES
46
(No Transcript)