PHARMACEUTICAL INDUSTRY - PowerPoint PPT Presentation
1 / 24
Title:
PHARMACEUTICAL INDUSTRY
Description:
PHARMACEUTICAL INDUSTRY Drug Discovery - natural sources, synthesis/modification biological properties - pharmacology, pharmacokinetics Preclinical Studies – PowerPoint PPT presentation
Number of Views:130
Avg rating:3.0/5.0
Title: PHARMACEUTICAL INDUSTRY
1
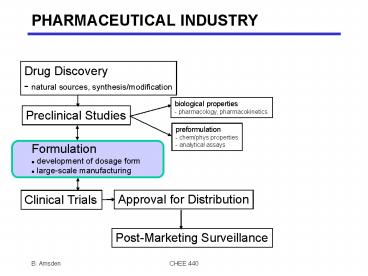
PHARMACEUTICAL INDUSTRY
Drug Discovery - natural sources,
synthesis/modification
biological properties - pharmacology,
pharmacokinetics
Preclinical Studies
preformulation - chem/phys properties -
analytical assays
- Formulation
- development of dosage form
- large-scale manufacturing
Clinical Trials
Approval for Distribution
Post-Marketing Surveillance
2
Definitions
- drug - Any substance or mixture of substances
manufactured, sold or represented for use in - a) the diagnosis, treatment, mitigation or
prevention of a disease, a disorder, an abnormal
physical state or the symptoms thereof in humans
or animals - b) restoring, correcting or modifying organic
functions in humans or animals - c) disinfection in premises in which food is
manufactured, prepared or kept - pharmaceutics - the area of study concerned with
the formulation, manufacture, stability, and
effectiveness of dosage forms - pharmacology - the science of the properties of
drugs and their effects on the body - pharmacokinetics - the study of the kinetics of
absorption, distribution, metabolism, and
excretion of drugs and their corresponding
pharmacologic response in animals/man - clinic - a facility or area where ambulatory
patients are seen for special study and treatment
3
Introduction
- Drugs seldom administered alone
- contain additional ingredients called excipients
- Need for dosage forms
- provide safe and accurate delivery
- protect drug from environmental and in vivo
degradation - provide rate-controlled action
- conceal bitter/salty taste, offensive odor
- allow for administration by the desired route
- Objective of dosage form design
- achieve a predictable therapeutic response to a
drug included in a formulation which is capable
of large scale manufacture with reproducible
product quality
4
Excipients
5
Routes of Administration
- Considering only systemic delivery, wherein the
objective is to get the drug into the blood
stream. There are essentially two classes of
delivery approaches - enteral
- oral (peroral), rectal, buccal and sublingual
- parenteral
- injection (s.c., i.v., i.m.)
- transdermal
- nasal
- pulmonary
6
Bioavailability
- extent of absorption and the rate at which an
administered dose reaches systemic circulation in
its active form
7
Absorption
- Affected by
- 1. Physiological factors
- route of administration
- drug distribution
- 2. Drug chemical physical properties
- dissolution rate (solids)
- hydrophilicity/hydrophobicity
8
Oral
9
Oral Absorption
10
Oral
- gastric emptying
- volume of gastric contents determines drug
- time dosage form/drug spends in stomach
influences absorption - liquids emptied faster than solids
- acids slow gastric emptying
- natural triglycerides inhibit gastric motility
- eating influences transit
11
Drug Absorption
- oral administration plasma concentration time
profile
12
Therapeutic Window
- therapeutic response is dependent on drug
achieving an adequate plasma concentration (Cp)
Cp
13
Oral
- advantages
- patient compliance
- cheap compared to other routes
- transit time is consistent among individuals
- disadvantages
- hepatic first-pass effect
- possible enzymatic degradation/acid degradation
- effect too slow for emergencies
- presence of food retards absorption
- short window of time for absorption
14
Rectal
- Rectal route
- lined with one or more layers of epithelial cells
- luminal side covered with mucus layer
- contains a small amount (1-3 ml) of fluid
- fluid has low buffering capacity
- abundantly vascularized
- drug absorption primarily by passive diffusion
- avoids some first pass clearance
15
Buccal and Sublingual
- Avoids exposure to GIT.
16
Parenteral
- i.v.
plasma concn
time after administration
17
Parenteral
- i.m. and s.c.
- not all drugs fully absorbed
- tissue more acidic than most tissues
- blood flow is important
- good supply of capillaries
- drug absorption function of diffusion rate
18
Transdermal
- rate limiting step is diffusion through stratum
corneum
19
Transdermal
Factors affecting absorption
20
Transdermal
- Limitations
- drug must be potent
- drug must be effective when delivered slowly over
a long period of time - benefits over existing methods?
- Drug qualifications
- narrow therapeutic window
- subject to extensive first-pass degradation
- taken many times/day
- unpleasant side-effects
21
Transdermally Delivered Drugs
22
Nasal
advantageous for drugs poorly absorbed orally for
some peptides and small molecules,
bioavailability comparable to injections drugs
lypressin, desmopressin, vitamin B-12,
progesterone, insulin, calcitonin, propanolol
external naris
23
Pulmonary
- - large contact surface (surface area gt 30 m2 )
- - extensive blood supply (2000 km of capillaries)
- - thin membrane separating air from blood
24
Conventional Dosage Forms